新技术在慢加急性肝衰竭全病程管理中的转化与落地
DOI: 10.12449/JCH250604
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:何恺负责文章撰写,李海负责文章构思、撰写及修订。
Translation and implementation of new technologies in the whole-course management of acute-on-chronic liver failure
-
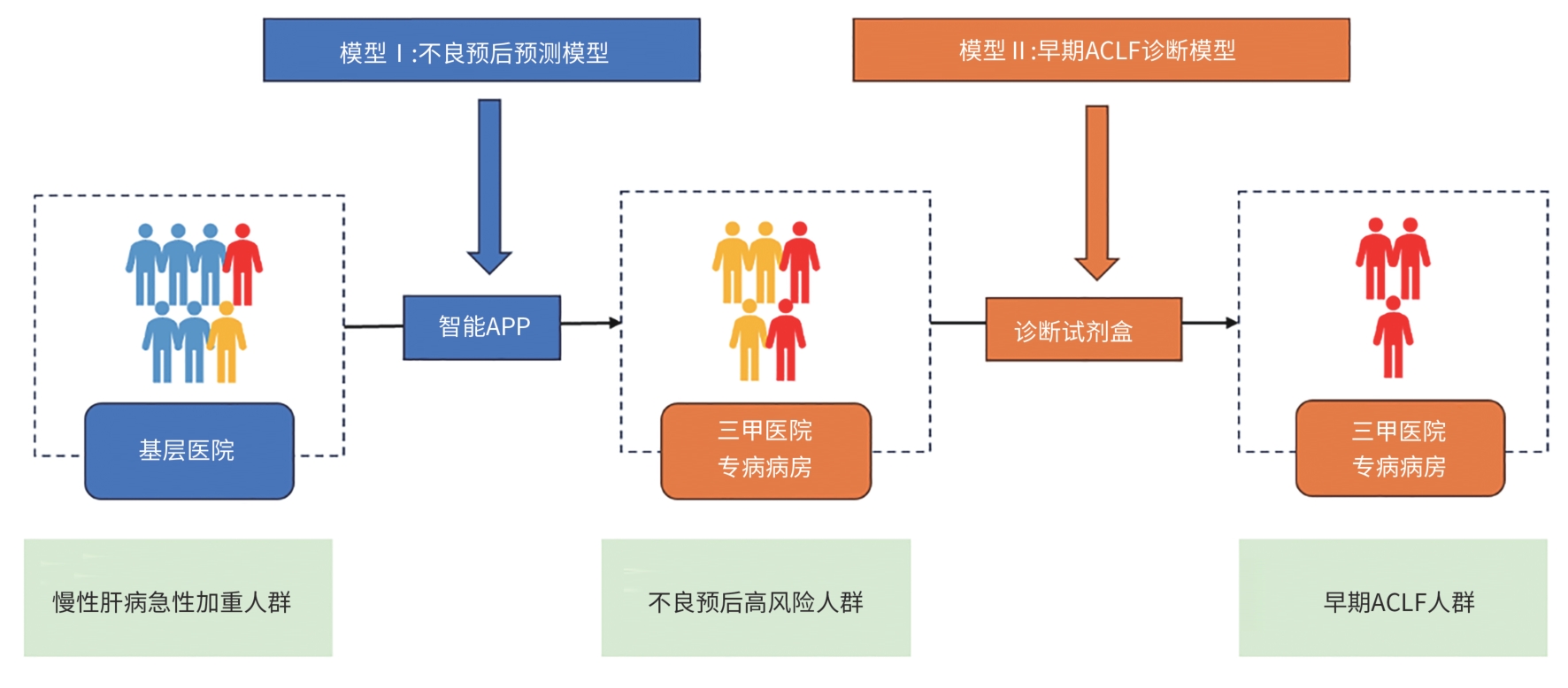
摘要: 慢加急性肝衰竭(ACLF)是在慢性肝病基础上出现的急性肝功能不全,具有较高病死率。为了改善ACLF患者的预后,需要尽可能地早期识别ACLF前期的患者,同时不断优化、创新疾病进展期诊疗方案。经过中国ACLF联盟10余年的研究,开发出针对ACLF器官衰竭的预警模型,并开创了早期高风险患者的基层转诊体系,现逐步进行的真实世界研究已验证了预警模型与临床的吻合度,在ACLF的早筛、早诊、早治方面做出了充分的贡献。对于进展期ACLF的治疗路径也在逐步扩展,从创新性药物的开发,到生物人工肝和干细胞的应用,都已在临床研究上取得了显著的进展,均有望在不远的将来正式落地。更高效的诊断体系与新颖的治疗方式配合,定会将国内ACLF的诊治能力推向全新的高峰。Abstract: Acute-on-chronic liver failure (ACLF) is a form of acute hepatic insufficiency that occurs in the context of a chronic liver disease, with a relatively high mortality rate. To improve the prognosis of ACLF patients, it is essential to early identify the patients with pre-ACLF and constantly optimize and innovate treatment regimens for the disease in the progressive stage. With more than 10 years of research, the Chinese CLIF consortium has developed an early warning model for ACLF and established a system for transferring high-risk patients to tertiary hospitals. At present, the real-world study has also confirmed the consistency between the early warning model and actual conditions in clinical practice, making contributions to the early screening, diagnosis, and treatment of ACLF. The treatment options for the progressive stage of ACLF are also expanding, from the development of innovative pharmaceuticals to the use of artificial liver support and stem cell therapy, and such treatment modalities have made significant achievements in clinical studies and are expected to be implemented in the near future. The development of a more efficient diagnostic system and novel treatment modalities has led to a significant improvement in the diagnosis and treatment of ACLF.
-
Key words:
- Acute-On-Chronic Liver Failure /
- Early Diagnosis /
- Therapeutics /
- Prognosis
-
[1] ARROYO V, MOREAU R, JALAN R. Acute-on-chronic liver failure[J]. N Engl J Med, 2020, 382( 22): 2137- 2145. DOI: 10.1056/nejmra1914900. [2] WANG X, YANG JH, ZHENG MY, et al. Advances in diagnostic criteria for acute-on-chronic liver failure[J]. Chin Gen Pract, 2023, 26( 7): 886- 892, 902. DOI: 10.12114/j.issn.1007-9572.2022.0493.王霞, 杨晋辉, 郑梦瑶, 等. 慢加急性肝衰竭诊断标准的研究进展[J]. 中国全科医学, 2023, 26( 7): 886- 892, 902. DOI: 10.12114/j.issn.1007-9572.2022.0493. [3] MEZZANO G, JUANOLA A, CARDENAS A, et al. Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis[J]. Gut, 2022, 71( 1): 148- 155. DOI: 10.1136/gutjnl-2020-322161. [4] ARROYO V, MOREAU R, KAMATH PS, et al. Acute-on-chronic liver failure in cirrhosis[J]. Nat Rev Dis Primers, 2016, 2: 16041. DOI: 10.1038/nrdp.2016.41. [5] PLAUTH M, BERNAL W, DASARATHY S, et al. ESPEN guideline on clinical nutrition in liver disease[J]. Clin Nutr, 2019, 38( 2): 485- 521. DOI: 10.1016/j.clnu.2018.12.022. [6] SCHÜTZ T, BECHSTEIN WO, NEUHAUS P, et al. Clinical practice of nutrition in acute liver failure: A European survey[J]. Clin Nutr, 2004, 23( 5): 975- 982. DOI: 10.1016/j.clnu.2004.03.005. [7] RABINOWICH L, WENDON J, BERNAL W, et al. Clinical management of acute liver failure: Results of an international multi-center survey[J]. World J Gastroenterol, 2016, 22( 33): 7595- 7603. DOI: 10.3748/wjg.v22.i33.7595. [8] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Gastroenterology, Chinese Medical Association. Clinical guidelines on nutrition in end-stage liver disease[J]. J Clin Hepatol, 2019, 35( 6): 1222- 1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010.中华医学会肝病学分会, 中华医学会消化病学分会. 终末期肝病临床营养指南[J]. 临床肝胆病杂志, 2019, 35( 6): 1222- 1230. DOI: 10.3969/j.issn.1001-5256.2019.06.010. [9] CHENG SQ. Progress in clinical application of liver-protecting drugs[J]. Her Med, 2004, 23( 12): 934- 937.程书权. 保肝药物的临床应用进展[J]. 医药导报, 2004, 23( 12): 934- 937. [10] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2024version)[J]. J Clin Hepatol, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 12): 2371- 2387. DOI: 10.12449/JCH241206. [11] XU WX, ZHU S, YANG L, et al. Safety and efficacy of double plasma molecular adsorption system with sequential low-volume plasma exchange in intermediate-stage hepatitis B virus-related acute-on-chronic liver failure[J]. J Med Virol, 2023, 95( 3): e28650. DOI: 10.1002/jmv.28650. [12] DAI XH, LIU J, SUN JL, et al. Application points and prospects of Li’s artificial liver support system[J]. Chin Clin Dr, 2020, 48( 11): 1274- 1278. DOI: 10.3969/j.issn.2095-8552.2020.11.004.戴霞红, 刘静, 孙建莉, 等. 李氏人工肝支持系统的应用要点及展望[J]. 中国临床医生杂志, 2020, 48( 11): 1274- 1278. DOI: 10.3969/j.issn.2095-8552.2020.11.004. [13] BAJAJ JS, O’LEARY JG, LAI JC, et al. Acute-on-chronic liver failure clinical guidelines[J]. Am J Gastroenterol, 2022, 117( 2): 225- 252. DOI: 10.14309/ajg.0000000000001595. [14] PIANO S, MAHMUD N, CARACENI P, et al. Mechanisms and treatment approaches for ACLF[J]. Liver Int, 2025, 45( 3): e15733. DOI: 10.1111/liv.15733. [15] CHOUDHURY A, KULKARNI AV, ARORA V, et al. Acute-on-chronic liver failure(ACLF): The‘Kyoto consensus’-steps from Asia[J]. Hepatol Int, 2025, 19( 1): 1- 69. DOI: 10.1007/s12072-024-10773-4. [16] TOMESCU D, POPESCU M, BIANCOFIORE G. Liver transplantation for acute-on-chronic liver failure[J]. Best Pract Res Clin Anaesthesiol, 2020, 34( 1): 25- 33. DOI: 10.1016/j.bpa.2019.12.001. [17] GUSTOT T, MOREAU R. Acute-on-chronic liver failure vs. traditional acute decompensation of cirrhosis[J]. J Hepatol, 2018, 69( 6): 1384- 1393. DOI: 10.1016/j.jhep.2018.08.024. [18] TAPPER EB, PARIKH ND. Diagnosis and management of cirrhosis and its complications: A review[J]. JAMA, 2023, 329( 18): 1589- 1602. DOI: 10.1001/jama.2023.5997. [19] GU WY, XU BY, ZHENG X, et al. Acute-on-chronic liver failure in China: Rationale for developing a patient registry and baseline characteristics[J]. Am J Epidemiol, 2018, 187( 9): 1829- 1839. DOI: 10.1093/aje/kwy083. [20] QIAO L, WANG XB, DENG GH, et al. Cohort profile: A multicentre prospective validation cohort of the Chinese Acute-on-Chronic Liver Failure(CATCH-LIFE) study[J]. BMJ Open, 2021, 11( 1): e037793. DOI: 10.1136/bmjopen-2020-037793. [21] ZHANG Y, TAN WT, WANG XB, et al. Metabolic biomarkers significantly enhance the prediction of HBV-related ACLF occurrence and outcomes[J]. J Hepatol, 2023, 79( 5): 1159- 1171. DOI: 10.1016/j.jhep.2023.07.011. [22] TREBICKA J, HERNAEZ R, SHAWCROSS DL, et al. Recent advances in the prevention and treatment of decompensated cirrhosis and acute-on-chronic liver failure(ACLF) and the role of biomarkers[J]. Gut, 2024, 73( 6): 1015- 1024. DOI: 10.1136/gutjnl-2023-330584. [23] PAN HN, HONG F, RADAEVA S, et al. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3[J]. Cell Mol Immunol, 2004, 1( 1): 43- 49. [24] KONG XN, FENG DC, MATHEWS S, et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: Therapeutic potential for the treatment of alcoholic liver disease[J]. J Gastroenterol Hepatol, 2013, 28( Suppl 1): 56- 60. DOI: 10.1111/jgh.12032. [25] XIANG XG, HWANG S, FENG DC, et al. Interleukin-22 in alcoholic hepatitis and beyond[J]. Hepatol Int, 2020, 14( 5): 667- 676. DOI: 10.1007/s12072-020-10082-6. [26] TUERXUN K, HE JY, IBRAHIM I, et al. Bioartificial livers: A review of their design and manufacture[J]. Biofabrication, 2022, 14( 3): 032003. DOI: 10.1088/1758-5090/ac6e86. [27] WANG YF, ZHENG Q, SUN Z, et al. Reversal of liver failure using a bioartificial liver device implanted with clinical-grade human-induced hepatocytes[J]. Cell Stem Cell, 2023, 30( 5): 617- 631. e 8. DOI: 10.1016/j.stem.2023.03.013. [28] LIU YW, DONG YT, WU XJ, et al. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: A systematic review and meta-analysis of randomized controlled clinical trials[J]. Stem Cell Res Ther, 2022, 13( 1): 204. DOI: 10.1186/s13287-022-02882-4. [29] TIAN SY, ZHOU X, ZHANG M, et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages[J]. Stem Cell Res Ther, 2022, 13( 1): 330. DOI: 10.1186/s13287-022-03010-y. [30] LIN BL, CHEN JF, QIU WH, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial[J]. Hepatology, 2017, 66( 1): 209- 219. DOI: 10.1002/hep.29189. [31] XU WX, LI YM, WANG L, et al. Efficacy and safety of combination treatment of double plasma molecular adsorption system and low volume plasma exchange for patients with hepatitis B virus related acute-on-chronic liver failure: A multicentre randomised controlled clinical trial[J]. BMJ Open, 2021, 11( 12): e047690. DOI: 10.1136/bmjopen-2020-047690. -



 PDF下载 ( 977 KB)
PDF下载 ( 977 KB)

 下载:
下载:


