泛素特异性蛋白酶在肝细胞癌中的作用
DOI: 10.12449/JCH250525
-
摘要: 肝细胞癌(HCC)是一种常见的原发性恶性肿瘤。近年来,泛素特异性蛋白酶(USP)在HCC中的作用引起了广泛关注。USP是一类关键的去泛素化酶,通过调节蛋白质的泛素化状态,影响多种生物学过程。研究发现,USP通过去泛素化多种肿瘤相关蛋白,参与调控细胞增殖、凋亡、迁移和侵袭等过程。此外,USP的异常表达与HCC患者的预后密切相关,可能作为潜在的生物标志物和治疗靶点。本文综述了USP在HCC中的研究进展,探讨了USP在HCC发生、发展及转移中的关键作用。深入了解USP在HCC中的作用机制,不仅有助于揭示HCC的发病机理,也为开发新的诊断工具和治疗策略提供了科学依据。未来的研究应进一步探索USP在HCC中的调控作用,以期为HCC的临床治疗提供更多有效手段。Abstract: Hepatocellular carcinoma (HCC) is a common primary malignant tumor. In recent years, the role of ubiquitin-specific proteases (USPs) in HCC has attracted widespread attention. USPs are a class of key deubiquitinating enzymes that affect a variety of biological processes by regulating the ubiquitination status of proteins. Studies have shown that USPs participate in the regulation of cell proliferation, apoptosis, migration, and invasion by deubiquitinating various tumor-related proteins. In addition, the abnormal expression of USPs is closely associated with the prognosis of HCC patients and may thus be used as potential biomarkers and therapeutic targets. This article reviews the research advances in USPs in HCC and explores their key roles in the development, progression, and metastasis of HCC. A deep understanding of the mechanism of action of USPs in HCC not only helps to reveal the pathogenesis of HCC, but also provides a scientific basis for developing new diagnostic tools and treatment strategies. Future research should further explore the regulatory effect of USPs in HCC, in order to provide more effective means for the clinical treatment of HCC.
-
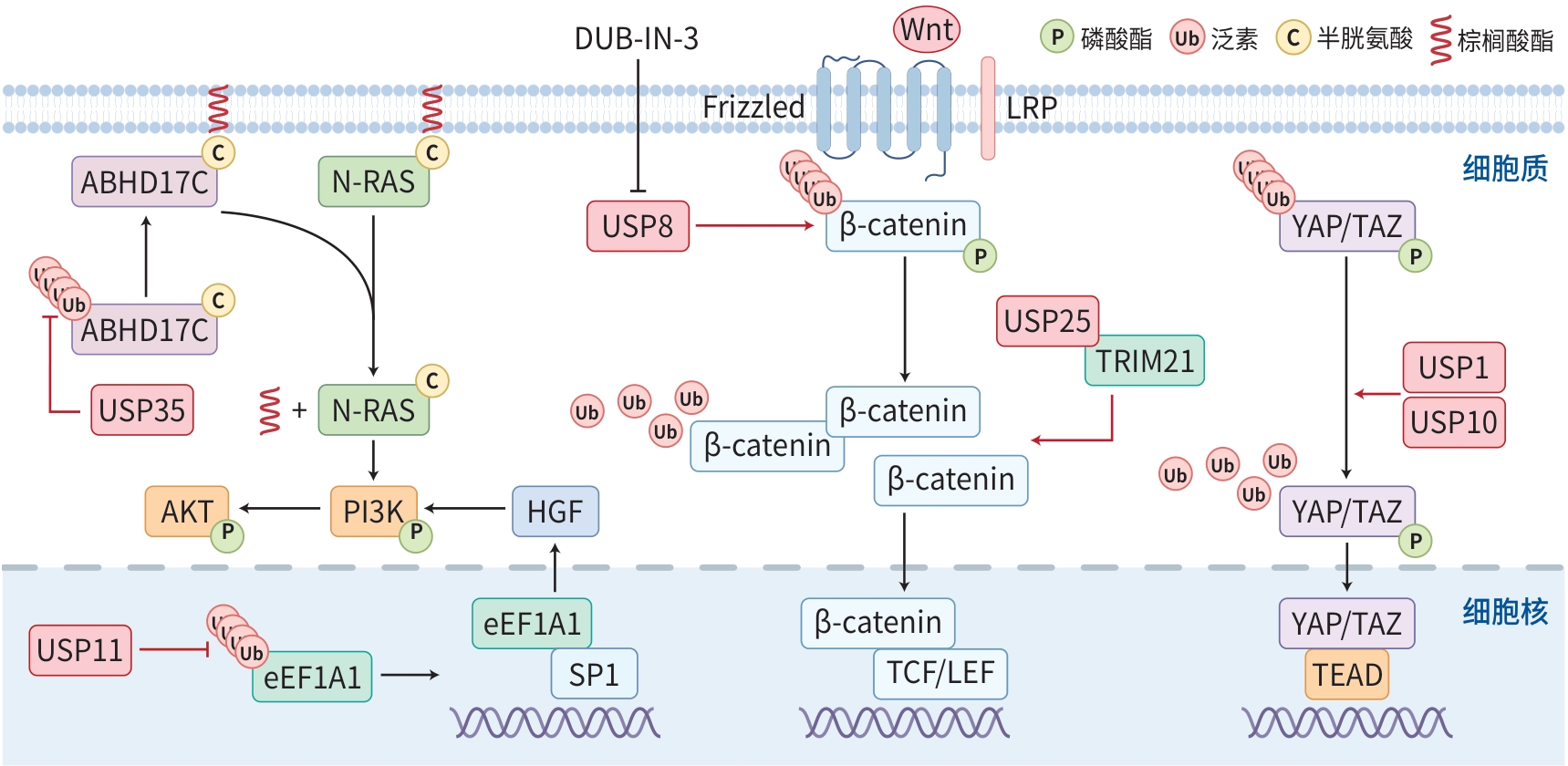
注: N-RAS,神经母细胞瘤RAS病毒癌基因同源物;HGF,肝细胞生长因子;TCF/LEF,T细胞因子/淋巴增强因子;TEAD,TEA结构域转录因子;LRP,低密度脂蛋白受体相关蛋白;Frizzled,卷曲蛋白。YAP/TAZ信号通路,USP1与USP10通过去泛素化和稳定YAP/TAZ来促进HCC的增殖,增强了YAP/TAZ在HCC细胞中的致癌功能[ 4, 14]。PI3K/AKT信号通路,USP35通过去泛素化稳定ABHD17C,从而激活N-RAS并通过PI3K/AKT信号通路促进HCC细胞的增殖、迁移和侵袭[ 30]。USP11阻止了eEF1A1的降解,促进SP1与HGF基因启动子的结合,增加HGF的表达,进而激活PI3K/AKT信号通路[ 15]。Wnt/β-catenin信号通路,USP8通过稳定β-catenin蛋白,从而激活Wnt/β-catenin信号通路[ 12]。USP25通过Wnt/β-catenin信号通路与TRIM21相互作用,促进HCC进展[ 26]。
图 1 DUB在HCC YAP/TAZ、PI3K/AKT和Wnt/β-catenin信号通路中的作用
Figure 1. The role of DUB in the YAP/TAZ, PI3K/AKT, and Wnt/β-catenin signaling pathways in hepatocellular carcinoma
表 1 HCC相关USP
Table 1. USP associated with hepatocellular carcinoma
USP 靶蛋白 信号通路 在HCC中的作用 参考文献 USP1 未明确 YAP/TAZ 生长、转移 [4] USP2a RAB1A 未明确 增殖、迁移、侵袭 [5] USP4 CypA MAPK 生长、迁移、侵袭 [6] TGF-βR1 TGF-β 侵袭、转移 [7] USP5 SLC7A11 未明确 铁死亡 [8] SLUG 未明确 增殖、转移、侵袭 [9] USP7 TRIP12 未明确 增殖、迁移、侵袭 [10] STAT3 未明确 增殖、转移 [11] USP8 未明确 Wnt/β-catenin 增殖、迁移、侵袭、铁死亡 [12] USP10 Smad4 TGF-β 转移 [13] YAP/TAZ YAP/TAZ 增殖、迁移 [14] USP11 eEF1A1 PI3K/AKT EMT、转移 [15] HIF-1α 未明确 糖酵解、增殖、转移 [16] NF90 未明确 增殖、转移 [17] USP12 未明确 MAPK 细胞凋亡 [18] USP13 TLR4 TLR4/MyD88/NF-κB 增殖、迁移、侵袭 [19] USP14 SQSTM1 未明确 自噬、增殖、侵袭、细胞凋亡 [20] HIF-1α 未明确 迁移、侵袭 [21] USP19 YAP Hippo 增殖、迁移 [22] USP21 MEK2 ERK 生长、增殖 [23] USP22 E2F6 AKT 生长 [24] HIF-1α 未明确 细胞干性、糖酵解 [25] USP25 TRIM21 Wnt/β-catenin 增殖、迁移、侵袭、EMT [26] USP27 SETD3 未明确 生长、增殖、迁移 [27] USP29 HIF-1α 未明确 糖酵解、耐药性 [28] USP35 PKM2 未明确 增殖、迁移、侵袭 [29] ABHD17C PI3K/AKT 生长、增殖、迁移 [30] USP39 SP1 未明确 增殖 [31] USP40 YAP 未明确 增殖、迁移 [32] USP46 MST1/YAP 未明确 增殖、转移 [33] 注:YAP/TAZ,Yes相关蛋白/PDZ结合基序转录共激活因子;RAB1A,Ras相关蛋白Rab-1A;CypA,亲环蛋白A;MAPK,丝裂原活化蛋白激酶;TGF-βR1,转化生长因子β受体1;SLC7A11,溶质载体家族7成员11;SLUG,锌指蛋白SNAI2;TRIP12,甲状腺激素受体相互作用蛋白12;STAT3,信号转导及转录激活因子3;Wnt/β-catenin,Wnt信号通路/β-连环蛋白;Smad4,SMAD家族成员4;eEF1A1,真核翻译延伸因子1A1;PI3K/AKT,磷脂酰肌醇3-激酶/蛋白激酶B;EMT,上皮-间充质转化;HIF-1α,缺氧诱导因子-1α;NF90,核因子90;MyD88,髓样分化因子88;SQSTM1,序列相似性家族62成员1;MEK2,丝裂原活化蛋白激酶激酶2;ERK,细胞外信号调节激酶;E2F6,E2F转录因子6;TRIM21,三结构域蛋白21;SETD3,SET结构域包含蛋白3;PKM2,丙酮酸激酶M2;ABHD17C,α/β水解酶结构域蛋白17C;SP1,特异性蛋白1;MST1,哺乳动物STE20样激酶1。
-
[1] SIEGEL RL, MILLER KD, WAGLE NS, et al. Cancer statistics, 2023[J]. CA A Cancer J Clinicians, 2023, 73( 1): 17- 48. DOI: 10.3322/caac.21763. [2] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [3] ÇETIN G, KLAFACK S, STUDENCKA-TURSKI M, et al. The ubiquitin-proteasome system in immune cells[J]. Biomolecules, 2021, 11( 1): 60. DOI: 10.3390/biom11010060. [4] LIU DY, LI QH, ZANG YF, et al. USP1 modulates hepatocellular carcinoma progression via the Hippo/TAZ axis[J]. Cell Death Dis, 2023, 14( 4): 264. DOI: 10.1038/s41419-023-05777-1. [5] XIONG B, HUANG JW, LIU Y, et al. Ubiquitin-specific protease 2a promotes hepatocellular carcinoma progression via deubiquitination and stabilization of RAB1A[J]. Cell Oncol(Dordr), 2021, 44( 2): 329- 343. DOI: 10.1007/s13402-020-00568-8. [6] LI TY, YAN B, MA Y, et al. Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin A stabilization and deubiquitination[J]. Cell Death Dis, 2018, 9( 2): 148. DOI: 10.1038/s41419-017-0182-5. [7] QIU C, LIU Y, MEI Y, et al. Ubiquitin-specific protease 4 promotes metastasis of hepatocellular carcinoma by increasing TGF-β signaling-induced epithelial-mesenchymal transition[J]. Aging(Albany NY), 2018, 10( 10): 2783- 2799. DOI: 10.18632/aging.101587. [8] YAN BK, GUO JX, WANG ZL, et al. The ubiquitin-specific protease 5 mediated deubiquitination of LSH links metabolic regulation of ferroptosis to hepatocellular carcinoma progression[J]. MedComm(2020), 2023, 4( 4): e337. DOI: 10.1002/mco2.337. [9] MENG J, AI XY, LEI YY, et al. USP5 promotes epithelial-mesenchymal transition by stabilizing SLUG in hepatocellular carcinoma[J]. Theranostics, 2019, 9( 2): 573- 587. DOI: 10.7150/thno.27654. [10] GEORGES A, MARCON E, GREENBLATT J, et al. Author correction: Identification and characterization of USP7 targets in cancer cells[J]. Sci Rep, 2019, 9: 15664. DOI: 10.1038/s41598-019-43448-4. [11] SU C, ZHANG HQ, MO J, et al. SP1-activated USP27X-AS1 promotes hepatocellular carcinoma progression via USP7-mediated AKT stabilisation[J]. Clin Transl Med, 2024, 14( 1): e1563. DOI: 10.1002/ctm2.1563. [12] TANG JN, LONG G, XIAO L, et al. USP8 positively regulates hepatocellular carcinoma tumorigenesis and confers ferroptosis resistance through β-catenin stabilization[J]. Cell Death Dis, 2023, 14( 6): 360. DOI: 10.1038/s41419-023-05747-7. [13] YUAN T, CHEN ZB, YAN FJ, et al. Deubiquitinating enzyme USP10 promotes hepatocellular carcinoma metastasis through deubiquitinating and stabilizing Smad4 protein[J]. Mol Oncol, 2020, 14( 1): 197- 210. DOI: 10.1002/1878-0261.12596. [14] ZHU H, YAN FJ, YUAN T, et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ[J]. Cancer Res, 2020, 80( 11): 2204- 2216. DOI: 10.1158/0008-5472.CAN-19-2388. [15] CHEN J, NING D, DU PC, et al. USP11 potentiates HGF/AKT signaling and drives metastasis in hepatocellular carcinoma[J]. Oncogene, 2024, 43( 2): 123- 135. DOI: 10.1038/s41388-023-02847-8. [16] QIAO LJ, HU WB, LI LZ, et al. USP11 promotes glycolysis by regulating HIF-1α stability in hepatocellular carcinoma[J]. J Cell Mol Med, 2024, 28( 2): e18017. DOI: 10.1111/jcmm.18017. [17] LUO CH, LU YY, FANG QL, et al. TRIM55 restricts the progression of hepatocellular carcinoma through ubiquitin-proteasome-mediated degradation of NF90[J]. Cell Death Discov, 2024, 10( 1): 441. DOI: 10.1038/s41420-024-02212-y. [18] LIU CS, LI XN, FENG G, et al. Downregulation of USP12 inhibits tumor growth via the p38/MAPK pathway in hepatocellular carcinoma[J]. Mol Med Rep, 2020, 22( 6): 4899- 4908. DOI: 10.3892/mmr.2020.11557. [19] GAO S, CHEN TX, LI LJ, et al. Hypoxia-inducible ubiquitin specific peptidase 13 contributes to tumor growth and metastasis via enhancing the toll-like receptor 4/myeloid differentiation primary response gene 88/nuclear factor-κB pathway in hepatocellular carcinoma[J]. Front Cell Dev Biol, 2020, 8: 587389. DOI: 10.3389/fcell.2020.587389. [20] ZHANG NN, ZHANG H, YANG XB, et al. USP14 exhibits high expression levels in hepatocellular carcinoma and plays a crucial role in promoting the growth of liver cancer cells through the HK2/AKT/P62 axis[J]. BMC Cancer, 2024, 24( 1): 237. DOI: 10.1186/s12885-024-12009-y. [21] LV C, WANG SL, LIN L, et al. USP14 maintains HIF1-α stabilization via its deubiquitination activity in hepatocellular carcinoma[J]. Cell Death Dis, 2021, 12( 9): 803. DOI: 10.1038/s41419-021-04089-6. [22] TIAN ZL, XU C, HE WX, et al. The deubiquitinating enzyme USP19 facilitates hepatocellular carcinoma progression through stabilizing YAP[J]. Cancer Lett, 2023, 577: 216439. DOI: 10.1016/j.canlet.2023.216439. [23] LI WJ, CUI KS, PROCHOWNIK EV, et al. The deubiquitinase USP21 stabilizes MEK2 to promote tumor growth[J]. Cell Death Dis, 2018, 9( 5): 482. DOI: 10.1038/s41419-018-0523-z. [24] JING TT, WANG BS, YANG ZJ, et al. Deubiquitination of the repressor E2F6 by USP22 facilitates AKT activation and tumor growth in hepatocellular carcinoma[J]. Cancer Lett, 2021, 518: 266- 277. DOI: 10.1016/j.canlet.2021.07.044. [25] LING SB, SHAN QN, ZHAN QF, et al. USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation[J]. Gut, 2020, 69( 7): 1322- 1334. DOI: 10.1136/gutjnl-2019-319616. [26] LIU YH, MA JJ, LU SM, et al. USP25 promotes hepatocellular carcinoma progression by interacting with TRIM21 via the Wnt/β-catenin signaling pathway[J]. Chin Med J(Engl), 2023, 136( 18): 2229- 2242. DOI: 10.1097/CM9.0000000000002714. [27] ZOU TT, WANG Y, DONG L, et al. Stabilization of SETD3 by deubiquitinase USP27 enhances cell proliferation and hepatocellular carcinoma progression[J]. Cell Mol Life Sci, 2022, 79( 1): 70. DOI: 10.1007/s00018-021-04118-9. [28] GAO RZ, BUECHEL D, KALATHUR RKR, et al. USP29-mediated HIF1α stabilization is associated with Sorafenib resistance of hepatocellular carcinoma cells by upregulating glycolysis[J]. Oncogenesis, 2021, 10( 7): 52. DOI: 10.1038/s41389-021-00338-7. [29] LV T, ZHANG B, JIANG CH, et al. USP35 promotes hepatocellular carcinoma progression by protecting PKM2 from ubiquitination-mediated degradation[J]. Int J Oncol, 2023, 63( 4): 113. DOI: 10.3892/ijo.2023.5561. [30] WANG LP, WANG JW, MA XQ, et al. USP35 promotes HCC development by stabilizing ABHD17C and activating the PI3K/AKT signaling pathway[J]. Cell Death Discov, 2023, 9( 1): 421. DOI: 10.1038/s41420-023-01714-5. [31] DONG X, LIU ZX, ZHANG EC, et al. USP39 promotes tumorigenesis by stabilizing and deubiquitinating SP1 protein in hepatocellular carcinoma[J]. Cell Signal, 2021, 85: 110068. DOI: 10.1016/j.cellsig.2021.110068. [32] MO HY, LI RT, YANG N, et al. USP40 promotes hepatocellular carcinoma progression through a YAP/USP40 positive feedback loop[J]. Cancer Lett, 2024, 589: 216832. DOI: 10.1016/j.canlet.2024.216832. [33] QIU YM, HUANG D, SHENG YL, et al. Deubiquitinating enzyme USP46 suppresses the progression of hepatocellular carcinoma by stabilizing MST1[J]. Exp Cell Res, 2021, 405( 1): 112646. DOI: 10.1016/j.yexcr.2021.112646. [34] HUANG P, WANG YH, ZHANG PF, et al. Ubiquitin-specific peptidase 1: Assessing its role in cancer therapy[J]. Clin Exp Med, 2023, 23( 7): 2953- 2966. DOI: 10.1007/s10238-023-01075-4. [35] CHEN SP, ZHU GQ, XING XX, et al. LncRNA USP2-AS1 promotes hepatocellular carcinoma growth by enhancing YBX1-mediated HIF1α protein translation under hypoxia[J]. Front Oncol, 2022, 12: 882372. DOI: 10.3389/fonc.2022.882372. [36] CAO YQ, XIA H, TAN XY, et al. Intratumoural microbiota: A new frontier in cancer development and therapy[J]. Signal Transduct Target Ther, 2024, 9( 1): 15. DOI: 10.1038/s41392-023-01693-0. [37] LU C, NING Z, WANG AM, et al. USP10 suppresses tumor progression by inhibiting mTOR activation in hepatocellular carcinoma[J]. Cancer Lett, 2018, 436: 139- 148. DOI: 10.1016/j.canlet.2018.07.032. [38] YE QW, ZHOU W, XU SJ, et al. Ubiquitin-specific protease 22 promotes tumorigenesis and progression by an FKBP12/mTORC1/autophagy positive feedback loop in hepatocellular carcinoma[J]. MedComm(2020), 2023, 4( 6): e439. DOI: 10.1002/mco2.439. [39] NING Z, GUO X, LIU XL, et al. USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma[J]. Nat Commun, 2022, 13( 1): 2187. DOI: 10.1038/s41467-022-29846-9. [40] GUO JH, ZHAO J. USP22-JMJD8 axis promotes Lenvatinib resistance in hepatocellular carcinoma[J]. Biochim Biophys Acta Mol Cell Res, 2024, 1871( 1): 119617. DOI: 10.1016/j.bbamcr.2023.119617. [41] ZENG K, XIE WW, WANG CY, et al. USP22 upregulates ZEB1-mediated VEGFA transcription in hepatocellular carcinoma[J]. Cell Death Dis, 2023, 14( 3): 194. DOI: 10.1038/s41419-023-05699-y. [42] CHANG YS, SU CW, CHEN SC, et al. Upregulation of USP22 and ABCC1 during sorafenib treatment of hepatocellular carcinoma contribute to development of resistance[J]. Cells, 2022, 11( 4): 634. DOI: 10.3390/cells11040634. [43] GAO HL, XI Z, DAI JW, et al. Drug resistance mechanisms and treatment strategies mediated by Ubiquitin-Specific Proteases(USPs) in cancers: New directions and therapeutic options[J]. Mol Cancer, 2024, 23( 1): 88. DOI: 10.1186/s12943-024-02005-y. [44] CHEN SS, ZHOU BH, HUANG W, et al. The deubiquitinating enzyme USP44 suppresses hepatocellular carcinoma progression by inhibiting Hedgehog signaling and PDL1 expression[J]. Cell Death Dis, 2023, 14( 12): 830. DOI: 10.1038/s41419-023-06358-y. [45] ZHOU WH, CHEN JF, WANG JG. Comprehensive prognostic and immunological analysis of Ubiquitin Specific Peptidase 28 in pan-cancers and identification of its role in hepatocellular carcinoma cell lines[J]. Aging(Albany NY), 2023, 15( 13): 6545- 6576. DOI: 10.18632/aging.204869. [46] LEI H, XU HZ, SHAN HZ, et al. Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia[J]. Nat Commun, 2021, 12( 1): 51. DOI: 10.1038/s41467-020-20259-0. [47] LU Y, GAO J, WANG PP, et al. Discovery of potent small molecule ubiquitin-specific protease 10 inhibitors with anti-hepatocellular carcinoma activity through regulating YAP expression[J]. Eur J Med Chem, 2024, 272: 116468. DOI: 10.1016/j.ejmech.2024.116468. [48] LARSSON P, OLSSON M, SARATHCHANDRA S, et al. Multi-omics analysis identifies repurposing bortezomib in the treatment of kidney-, nervous system-, and hematological cancers[J]. Sci Rep, 2024, 14( 1): 18576. DOI: 10.1038/s41598-024-62339-x. -



 PDF下载 ( 983 KB)
PDF下载 ( 983 KB)

 下载:
下载:


