ALBI、EZ-ALBI及PALBI评分对HCV相关肝细胞癌患者中期预后的预测价值分析
DOI: 10.12449/JCH250518
Value of albumin-bilirubin, easy albumin-bilirubin, and platelet-albumin-bilirubin scores in predicting the prognosis of patients with HCV-associated hepatocellular carcinoma
-
摘要:
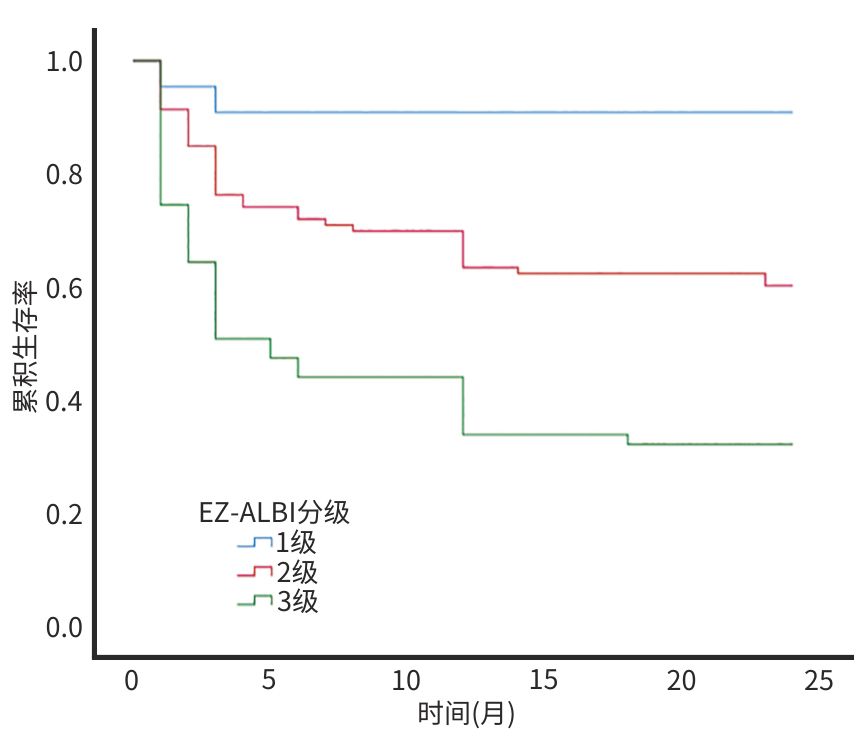
目的 探讨白蛋白-胆红素(ALBI)、简易白蛋白-胆红素(EZ-ALBI)及血小板-白蛋白-胆红素(PALBI)评分对HCV相关肝细胞癌(HCV-HCC)患者2年生存期的预测价值。 方法 回顾性分析2020年1月—2022年1月于昆明市第三人民医院住院治疗的174例HCV-HCC患者临床资料,随访时间为入院后2年。根据随访结果将患者分为生存组(n=95)和死亡组(n=79)。计量资料两组间比较采用成组t检验或Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用单因素和多因素Cox比例风险回归模型分析HCV-HCC患者生存的影响因素。通过Kaplan-Meier法绘制生存曲线,分析不同EZ-ALBI分级HCV-HCC患者的2年生存率,并使用Log-rank检验进行组间比较。 结果 生存组与死亡组患者比较,PLT、AST、TBil、Alb、AFP、前白蛋白、凝血酶原时间、国际标准化比值、PALBI评分、ALBI评分、EZ-ALBI评分、终末期肝病模型(MELD)评分、HCV基因分型、腹腔积液、血管侵犯差异均有统计学意义(P值均<0.05)。单因素Cox回归分析结果显示,AST、Alb、AFP、ALBI评分、EZ-ALBI评分、PALBI评分、MELD评分、巴塞罗那临床肝癌分期和腹腔积液是患者生存的影响因素(P值均<0.05);进一步多因素Cox回归分析结果显示,EZ-ALBI评分(HR=1.850,95%CI:1.054~3.247,P=0.032)和腹腔积液(HR=1.993,95%CI:1.030~3.858,P=0.041)是HCV-HCC患者生存的独立危险因素。生存曲线分析结果显示,EZ-ALBI 1级、2级、3级患者的2年生存率分别为90.9%、60.2%和32.2%,不同EZ-ALBI分级患者累积生存率比较,差异有统计学意义(χ2=26.294,P<0.001)。 结论 EZ-ALBI评分及有无腹腔积液可作为HCV-HCC患者生存情况的预测指标。 Abstract:Objective To investigate the value of albumin-bilirubin (ALBI), easy albumin-bilirubin (EZ-ALBI), and platelet-albumin-bilirubin (PALBI) scores in predicting 2-year survival in patients with HCV-associated hepatocellular carcinoma (HCV-HCC). Methods A retrospective analysis was performed for the clinical data of 174 patients with HCV-HCC who were admitted to The Third People’s Hospital of Kunming from January 2020 to January 2022, and the patients were followed up till 2 years after admission. According to the follow-up results, the patients were divided into survival group with 95 patients and death group with 79 patients. The independent-samples t test or the Mann-Whitney U test was used for comparison of continuous data between two groups, and the chi-square test was used for comparison of categorical data between two groups. Univariate and multivariate Cox proportional-hazards regression model analyses were used to investigate the influencing factors for the survival of HCV-HCC patients. The Kaplan-Meier method was used to plot survival curves and analyze the 2-year survival rate of HCV-HCC patients with different EZ-ALBI grades, and the log-rank test was used for comparison between groups. Results There were significant differences between the survival group and the death group in platelet count, aspartate aminotransferase (AST), total bilirubin, albumin (Alb), alpha-fetoprotein (AFP), prealbumin, prothrombin time, international normalized ratio, PALBI score, ALBI score, EZ-ALBI score, Model for End-Stage Liver Disease (MELD) score, HCV genotype, peritoneal effusion, and vascular invasion (all P<0.05). The univariate Cox regression analysis showed that AST, Alb, AFP, ALBI score, EZ-ALBI score, PALBI score, MELD score, Barcelona Clinic Liver Cancer Staging, and peritoneal effusion were influencing factors for the survival of patients (all P<0.05), and the multivariate Cox regression analysis showed that EZ-ALBI score (hazard ratio [HR]=1.850, 95% confidence interval [CI]: 1.054 — 3.247, P=0.032) and peritoneal effusion (HR=1.993, 95%CI: 1.030 — 3.858, P=0.041) were independent risk factors for the survival of HCV-HCC patients. The survival curve analysis showed that the patients with EZ-ALBI grade 1/2/3 had a 2-year survival rate of 90.9%, 60.2%, and 32.2%, respectively, and there was a significant difference in cumulative survival rate between the patients with different EZ-ALBI grades (χ2=26.294, P<0.001). Conclusion EZ-ALBI score and the presence or absence of peritoneal effusion can be used as predictors of the survival of HCV-HCC patients. -
Key words:
- Hepacivirus /
- Carcinoma, Hepatocellular /
- ALBI Score /
- EZ-ALBI Score /
- PALBI Score /
- Prognosis
-
表 1 两组患者基本资料
Table 1. Basic information of two groups of patients
项目 生存组(n=95) 死亡组(n=79) 统计值 P值 男/女(例) 76/19 58/21 χ2=1.056 0.304 年龄(岁) 55.13±8.57 54.19±6.37 t=-0.804 0.423 血管侵犯(有/无,例) 41/54 52/27 χ2=8.905 0.003 HCV基因分型(3型/其他,例) 52/43 55/24 χ2=4.035 0.045 腹腔积液(有/无,例) 58/37 68/11 χ2=13.520 <0.001 BCLC分期(例) χ2=9.001 0.061 0期 17 4 A期 20 19 B期 37 28 C期 6 7 D期 15 21 PLT(×109/L) 90(68~131) 102(59~153) Z=-0.846 <0.001 ALT(U/L) 34(20~67) 36(28~85) Z=-1.291 0.197 AST(U/L) 50(35~100) 86(48~148) Z=-3.278 0.001 PT(s) 15.4(14.5~17.1) 16.7(15.6~18.2) Z=-3.691 <0.001 TBil(μmol/L) 23.5(17.4~42.8) 41.8(23.3~93.9) Z=-3.651 <0.001 PAB(mg/L) 100.00(61.40~141.05) 71.45(49.45~107.05) Z=-3.498 <0.001 Alb(g/L) 33.13±6.75 28.79±5.39 t=4.723 <0.001 GGT(U/L) 112.30(42.25~279.25) 132.35(65.00~275.25) Z=-1.063 0.288 AFP(log10 ng/mL) 1.56(0.84~3.36) 2.88(1.33~4.19) Z=-2.595 0.009 CREA(μmol/L) 63.00(49.25~77.25) 76.72(65.00~83.00) Z=-0.642 0.521 INR 1.36(1.16~1.43) 1.43(1.26~1.52) Z=-2.977 0.003 HCV RNA(有/无,例) 32/63 36/43 χ2=2.559 0.110 PALBI评分(分) -2.109(-2.502~-1.822) -1.714(-2.054~-1.317) Z=-4.944 <0.001 ALBI评分(分) -2.58(-3.14~-2.17) -2.16(-2.53~-1.88) Z=-4.578 <0.001 EZ-ALBI评分(分) -27.85(-33.54~-22.68) -22.17(-27.02~-17.51) Z=-4.959 <0.001 MELD评分(分) 12.55(9.02~13.72) 15.05(10.98~17.69) Z=-3.677 <0.001 饮酒史(有/无,例) 67/28 52/27 χ2=0.441 0.506 吸毒史(有/无,例) 46/49 38/41 χ2=0.002 0.966 手术方式(例) χ2=2.157 0.324 介入 77 57 外科 3 5 未处理 15 17 注:BCLC分期,巴塞罗那临床肝癌分期;PT,凝血酶原时间;PAB,前白蛋白;CREA,肌酐;INR,国际标准化比值。
表 2 单因素Cox比例风险回归分析HCV-HCC患者生存的影响因素
Table 2. Single factor Cox proportional hazards regression analysis of factors affecting survival in HCV-HCC patients
项目 HR 95%CI P值 性别(男 vs 女) 1.284 0.779~2.116 0.326 年龄(岁) 0.989 0.961~1.018 0.448 PLT(×109/L) 1.002 1.000~1.005 0.080 ALT(U/L) 1.001 0.999~1.003 0.323 AST(>40 U/L vs ≤40 U/L) 2.017 1.149~3.541 0.015 PT(s) 1.029 0.981~1.078 0.240 Alb(>34 g/L vs ≤34 g/L) 0.925 0.982~0.960 <0.001 AFP(log10 ng/mL) 1.226 1.060~1.418 0.006 ALBI评分(分) 2.181 1.401~3.397 0.001 EZ-ALBI评分(分) 2.496 1.708~3.646 <0.001 PALBI评分(分) 2.908 1.886~4.482 <0.001 基因分型(3型 vs 其他) 1.613 0.998~2.608 0.051 血管侵犯(有 vs 无) 1.538 0.986~2.398 0.057 BCLC分期(0期 vs A期 vs B期 vs C期 vs D期) 1.199 1.009~1.426 0.039 手术方式(介入 vs 外科 vs 未处理) 1.168 0.898~1.519 0.247 MELD评分(分) 1.463 1.125~1.902 0.005 腹腔积液(有 vs 无) 2.819 1.490~5.333 0.001 -
[1] LEMON SM, MCGIVERN DR. Is hepatitis C virus carcinogenic?[J]. Gastroenterology, 2012, 142( 6): 1274- 1278. DOI: 10.1053/j.gastro. 2012.01.045. [2] ENGELSKIRCHER SA, CHEN PC, STRUNZ B, et al. Impending HCC diagnosis in patients with cirrhosis after HCV cure features a natural killer cell signature[J]. Hepatology, 2024, 80( 1): 202- 222. DOI: 10.1097/HEP.0000000000000804. [3] EMAMAULLEE JA, BRAL M, MEEBERG G, et al. HCV eradication with direct-acting antivirals does not impact HCC progression on the waiting list or HCC recurrence after liver transplantation[J]. Can J Gastroenterol Hepatol, 2019, 2019: 2509059. DOI: 10.1155/2019/2509059. [4] ZHANG Y, CHEN LM, HE M. Hepatitis C virus in mainland China with an emphasis on genotype and subtype distribution[J]. Virol J, 2017, 14( 1): 41. DOI: 10.1186/s12985-017-0710-z. [5] PENG J, LU YJ, LIU WY, et al. Genotype distribution and molecular epidemiology of hepatitis C virus in Hubei, Central China[J]. PLoS One, 2015, 10( 9): e0137059. DOI: 10.1371/journal.pone.0137059. [6] FALADE-NWULIA O, SUAREZ-CUERVO C, NELSON DR, et al. Oral direct-acting agent therapy for hepatitis C virus infection: A systematic review[J]. Ann Intern Med, 2017, 166( 9): 637- 648. DOI: 10.7326/M16-2575. [7] van der MEER AJ, VELDT BJ, FELD JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis[J]. JAMA, 2012, 308( 24): 2584- 2593. DOI: 10.1001/jama.2012.144878. [8] SEMMLER G, MEYER EL, KOZBIAL K, et al. HCC risk stratification after cure of hepatitis C in patients with compensated advanced chronic liver disease[J]. J Hepatol, 2022, 76( 4): 812- 821. DOI: 10.1016/j.jhep.2021.11.025. [9] KHATUN M, RAY R, RAY RB. Hepatitis C virus associated hepatocellular carcinoma[J]. Adv Cancer Res, 2021, 149: 103- 142. DOI: 10.1016/bs.acr.2020.10.003. [10] YANG CK, XU JM, WANG HX, et al. Predictive values of ALBI and EZ-ALBI scores for early survival of recipients with liver failure after liver transplantation[J]. Organ Transplant, 2022, 13( 5): 611- 617. DOI: 10.3969/j.issn.1674-7445.2022.05.010.杨程凯, 许嘉绵, 王华翔, 等. ALBI和EZ-ALBI评分对肝衰竭肝移植术后早期生存的预测价值[J]. 器官移植, 2022, 13( 5): 611- 617. DOI: 10.3969/j.issn.1674-7445.2022.05.010. [11] CHEN Y, SHI RJ, ZHANG FL, et al. Clinical application status of PALBI score in patients with liver cancer[J]. Chin J Gastroenterol Hepatol, 2022, 31( 12): 1342- 1345, 1352. DOI: 10.3969/j.issn.1006-5709.2022.12.005.陈怡, 施荣杰, 张凤莲, 等. PALBI评分在肝癌患者中的临床应用现状[J]. 胃肠病学和肝病学杂志, 2022, 31( 12): 1342- 1345, 1352. DOI: 10.3969/j.issn.1006-5709.2022.12.005. [12] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [13] HANSMANN J, EVERS MJ, BUI JT, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma[J]. J Vasc Interv Radiol, 2017, 28( 9): 1224- 1231. DOI: 10.1016/j.jvir.2017.05.020. [14] KARIYAMA K, NOUSO K, HIRAOKA A, et al. EZ-ALBI score for predicting hepatocellular carcinoma prognosis[J]. Liver Cancer, 2020, 9( 6): 734- 743. DOI: 10.1159/000508971. [15] KAMATH PS, WIESNER RH, MALINCHOC M, et al. A model to predict survival in patients with end-stage liver disease[J]. Hepatology, 2001, 33( 2): 464- 470. DOI: 10.1053/jhep.2001.22172. [16] YANG M, PARIKH ND, LIU HX, et al. Incidence and risk factors of hepatocellular carcinoma in patients with hepatitis C in China and the United States[J]. Sci Rep, 2020, 10( 1): 20922. DOI: 10.1038/s41598-020-77515-y. [17] LI F, LI B, ZHU QY. Research progress on the epidemiological characteristics and diagnosis of hepatitis C in China[J]. Int J Virol, 2023, 30( 6): 509- 511. DOI: 10.3760/cma.j.issn.1673-4092.2023.06.014.黎锋, 李博, 朱秋映. 中国丙肝流行特征与诊断研究进展[J]. 国际病毒学杂志, 2023, 30( 6): 509- 511. DOI: 10.3760/cma.j.issn.1673-4092.2023.06.014. [18] PARKIN DM, BRAY F, FERLAY J, et al. Global cancer statistics, 2002[J]. CA Cancer J Clin, 2005, 55( 2): 74- 108. DOI: 10.3322/canjclin.55.2.74. [19] YANG S. Antiviral treatment for hepatitis C and the prevention and treatment for liver cancer[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2024, 18( 1): 64. DOI: 10.3877/cma.j.issn.1674-1358.2024.01.011.杨松. 丙型肝炎抗病毒治疗与肝癌防治[J/CD]. 中华实验和临床感染病杂志(电子版), 2024, 18( 1): 64. DOI: 10.3877/cma.j.issn.1674-1358.2024.01.011. [20] XU SS, QIU LX, LIU YL, et al. Risk factors and predictive models for liver cancer after sustained virologic response in hepatitis C[J]. J Clin Hepatol, 2024, 40( 6): 1259- 1263. DOI: 10.12449/JCH240629.许姗姗, 仇丽霞, 柳雅立, 等. 丙型肝炎持续病毒学应答后肝癌发生的危险因素及预测模型[J]. 临床肝胆病杂志, 2024, 40( 6): 1259- 1263. DOI: 10.12449/JCH240629. [21] JIA YY, ZOU X, YUE W, et al. The distribution of hepatitis C viral genotypes shifted among chronic hepatitis C patients in Yunnan, China, between 2008-2018[J]. Front Cell Infect Microbiol, 2023, 13: 1092936. DOI: 10.3389/fcimb.2023.1092936. [22] WANG YJ, SONG HY, XING LJ, et al. Pathogenesis of hepatocellular carcinoma induced by HBV and HCV infection[J]. J Clin Hepatol, 2017, 33( 7): 1381- 1386. DOI: 10.3969/j.issn.1001-5256.2017.07.040.王月姣, 宋海燕, 邢练军, 等. HBV/HCV感染诱导肝细胞癌的病理机制[J]. 临床肝胆病杂志, 2017, 33( 7): 1381- 1386. DOI: 10.3969/j.issn.1001-5256.2017.07.040. [23] DENG SQ, ZHONG WF, CHEN W, et al. Hepatitis C viral load and mother-to-child transmission: A systematic review and meta-analysis[J]. J Gastroenterol Hepatol, 2023, 38( 2): 177- 186. DOI: 10.1111/jgh.15998. [24] REIG M, FORNER A, RIMOLA J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update[J]. J Hepatol, 2022, 76( 3): 681- 693. DOI: 10.1016/j.jhep.2021.11.018. [25] CAMPANI C, BAMBA-FUNCK J, CAMPION B, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab[J]. Liver Int, 2023, 43( 3): 708- 717. DOI: 10.1111/liv.15487. [26] DEMIRTAS CO, D’ALESSIO A, RIMASSA L, et al. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma[J]. JHEP Rep, 2021, 3( 5): 100347. DOI: 10.1016/j.jhepr.2021.100347. [27] JOHNSON PJ, BERHANE S, KAGEBAYASHI C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade[J]. J Clin Oncol, 2015, 33( 6): 550- 558. DOI: 10.1200/JCO.2014.57.9151. [28] ANTKOWIAK M, GABR A, DAS A, et al. Prognostic role of albumin, bilirubin, and ALBI scores: Analysis of 1000 patients with hepatocellular carcinoma undergoing radioembolization[J]. Cancers(Basel), 2019, 11( 6): 879. DOI: 10.3390/cancers11060879. [29] HO SY, LIU PH, HSU CY, et al. Comparison of four albumin-based liver reserve models(ALBI/EZ-ALBI/PALBI/PAL) against MELD for patients with hepatocellular carcinoma undergoing transarterial chemoembolization[J]. Cancers(Basel), 2023, 15( 7): 1925. DOI: 10. 3390/cancers15071925. [30] NI JY, FANG ZT, AN C, et al. Comparison of albumin-bilirubin grade, platelet-albumin-bilirubin grade and Child-Turcotte-Pugh class for prediction of survival in patients with large hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation[J]. Int J Hyperthermia, 2019, 36( 1): 841- 853. DOI: 10.1080/02656736.2019.1646927. [31] SONOHARA F, YAMADA S, TANAKA N, et al. Comparison of non-invasive liver reserve and fibrosis models: Implications for surgery and prognosis for hepatocellular carcinoma[J]. Hepatol Res, 2019, 49( 11): 1305- 1315. DOI: 10.1111/hepr.13400. [32] MÜLLER L, HAHN F, MÄHRINGER-KUNZ A, et al. Prevalence and clinical significance of clinically evident portal hypertension in patients with hepatocellular carcinoma undergoing transarterial chemoembolization[J]. United European Gastroenterol J, 2022, 10( 1): 41- 53. DOI: 10.1002/ueg2.12188. [33] MÜLLER L, BENDER D, GAIRING SJ, et al. Amount of ascites impacts survival in patients with hepatocellular carcinoma undergoing transarterial chemoembolization advocating for volumetric assessment[J]. Sci Rep, 2024, 14( 1): 16550. DOI: 10.1038/s41598-024-67312-2. [34] HO SY, YUAN MH, LIU PH, et al. Cryptogenic hepatocellular carcinoma: Characteristics, outcome, and prognostic role of albumin-bilirubin(ALBI) grade vs easy ALBI grade[J]. Scand J Gastroenterol, 2023, 58( 1): 61- 69. DOI: 10.1080/00365521.2022.2098052. [35] HO SY, LIU PH, HSU CY, et al. Easy albumin-bilirubin score as a new prognostic predictor in hepatocellular carcinoma[J]. Hepatol Res, 2021, 51( 11): 1129- 1138. DOI: 10.1111/hepr.13671. -



 PDF下载 ( 886 KB)
PDF下载 ( 886 KB)

 下载:
下载:


