单用卡维地洛与联合内镜下静脉曲张套扎术对肝硬化食管胃静脉曲张破裂出血二级预防的效果比较
DOI: 10.12449/JCH250515
Effectiveness of carvedilol alone versus carvedilol combined with endoscopic variceal ligation in secondary prevention of gastroesophageal variceal bleeding in patients with liver cirrhosis
-
摘要:
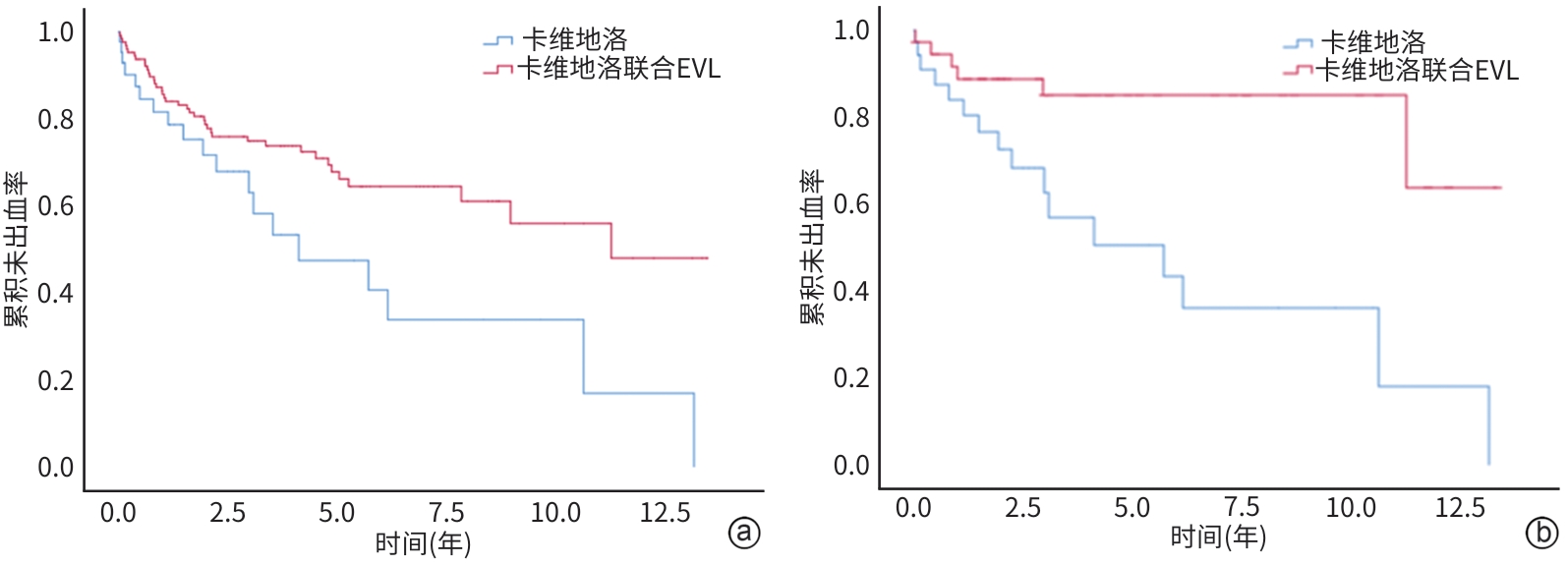
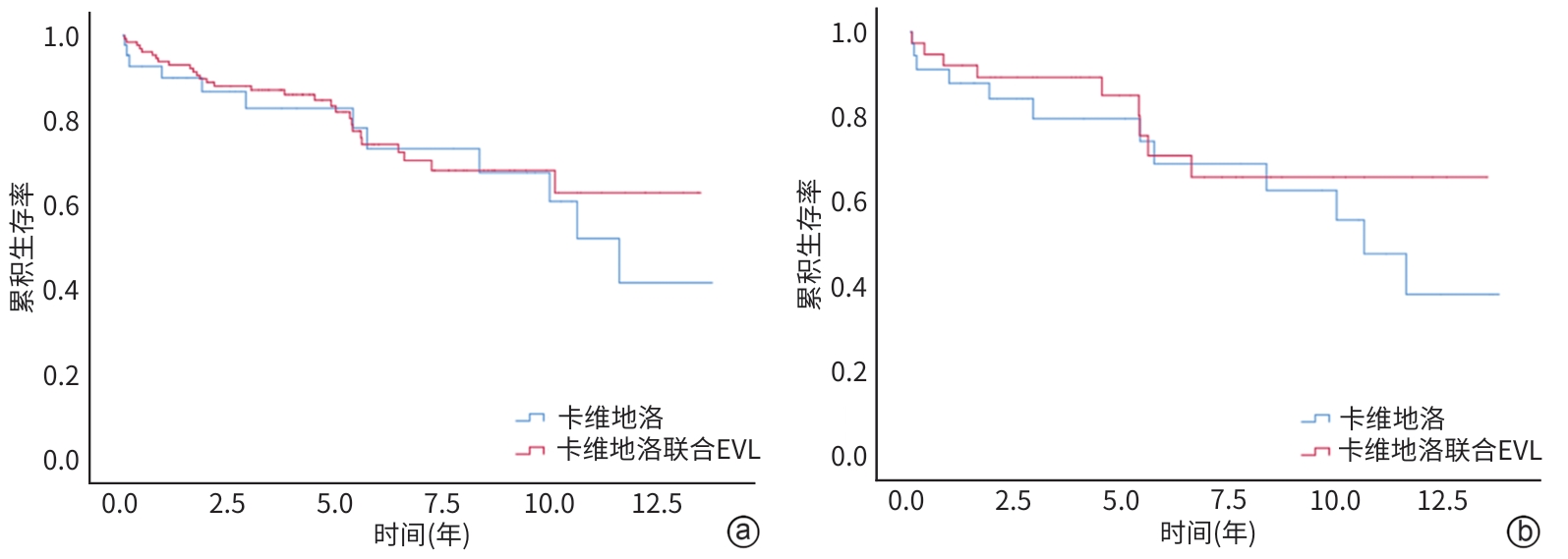
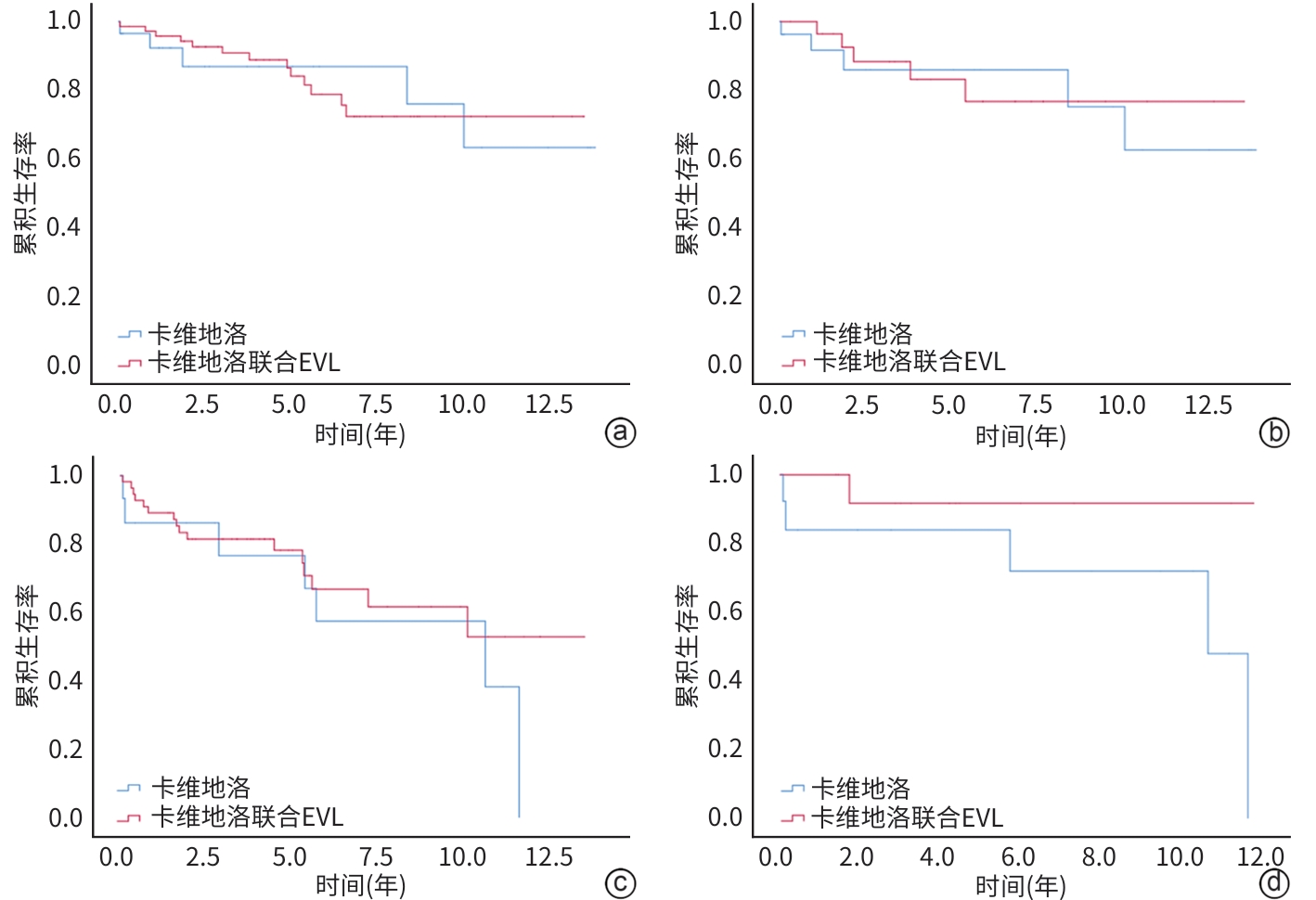
目的 旨在通过对比单用卡维地洛与卡维地洛联合内镜下静脉曲张套扎术(endoscopic variceal ligation,EVL)预防食管胃静脉再出血的治疗效果,为临床治疗提供策略。 方法 回顾性选取2010年10月—2023年6月于山东大学附属省立医院治疗,单用卡维地洛或卡维地洛联合EVL预防食管胃静脉曲张再出血的178例患者,分为单用卡维地洛组(n=47)和联合治疗组(n=131);收集两组患者再出血及死亡情况。符合正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。Cox比例风险模型用于单变量和多变量分析。采用Kaplan-Meier方法估计再出血和死亡的累积发生率。通过倾向性评分匹配(PSM)对卡维地洛组和联合治疗组进行匹配,使得两组患者基线相似,减小选择偏差并增强因果推断的可信度。 结果 联合治疗组再出血率显著低于卡维地洛组(10年累积发生率:29.8% vs 36.2%,HR=0.505,95%CI:0.292~0.847,P=0.015),但两组在肝脏相关死亡率方面差异无统计学意义(10年累积发生率:21.3% vs 21.4%,HR=0.799,95%CI:0.406~1.578,P=0.518),PSM分析结果进一步验证了上述结论的稳定性。Cox回归分析显示,肌酐是影响再出血(HR=1.004,95%CI:1.001~1.008,P=0.011)和肝脏相关死亡率(HR=1.004,95%CI:1.001~1.007,P=0.019)的独立危险因素。 结论 卡维地洛联用EVL预防食管胃静脉曲张出血效果优于卡维地洛单用。 Abstract:Objective To compare the therapeutic effects of carvedilol alone and carvedilol combined with endoscopic variceal ligation (EVL) in the prevention of re-bleeding from gastroesophageal varices, and to provide strategies for clinical treatment. Methods We retrospectively included 178 patients who had received carvedilol alone or carvedilol plus EVL to prevent gastroesophageal variceal re-hemorrhage from October 2010 to June 2023. They were divided into carvedilol alone group (47 cases) and carvedilol+EVL group (131 cases). Between-group comparisons were conducted using the paired t test for normally distributed continuous data, the Mann-Whitney U test for non-normally distributed continuous data, and the chi-square test for categorical data. A Cox proportional hazards model was employed for univariable and multi-variable analyses. The cumulative incidence rates of re-bleeding and mortality were estimated using the Kaplan-Meier method. The baseline characteristics of the two groups were matched through propensity score matching (PSM) to reduce selection bias and enhance the credibility of causal inference. Results The re-bleeding rate of the carvedilol+EVL group was significantly lower than that of the carvedilol alone group (10-year cumulative incidence: 29.8% vs 36.2%, hazard ratio [HR]=0.505, 95% confidence interval [CI]: 0.292 — 0.847, P=0.015). There was no significant difference in liver-related mortality (10-year cumulative incidence: 21.3% vs 21.4%, HR=0.799, 95%CI: 0.406 — 1.578, P=0.518). The results were stable with PSM analysis. The Cox regression analysis revealed that creatinine was an independent risk factor affecting re-bleeding (HR=1.004, 95%CI: 1.001 — 1.008, P=0.011) and liver-related mortality (HR=1.004, 95%CI: 1.001 — 1.007, P=0.019). Conclusion Carvedilol combined with EVL is better than carvedilol alone in the prevention of gastroesophageal variceal re-bleeding. -
表 1 两组患者的基线数据比较
Table 1. Demographic and baseline data for all patients
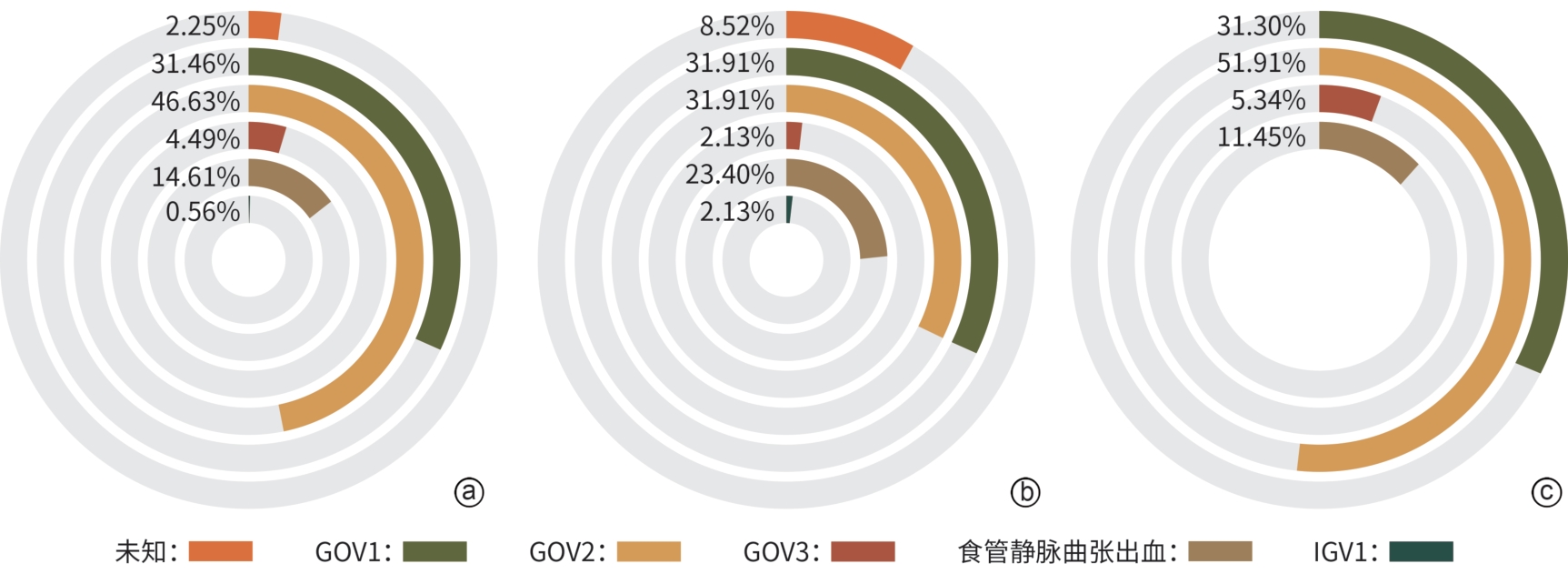
项目 卡维地洛组(n=47) 联合治疗组(n=131) 统计值 P值 年龄(岁) 51.06±12.71 53.76±13.51 t=-1.193 0.986 男/女(例) 32/15 82/49 χ2=0.453 0.501 脾栓塞或脾切除(无/有,例) 37/10 118/13 χ2=3.962 0.047 腹水(无/有,例) 30/17 65/66 χ2=2.807 0.094 肝性脑病(无/有,例) 47/0 129/2 χ2=0.726 0.394 肝硬化病因 χ2=6.646 0.248 乙型肝炎[例(%)] 24(51.1) 76(58.0) 丙型肝炎[例(%)] 3(6.4) 7(5.3) 酒精性肝病[例(%)] 9(19.1) 27(20.6) 胆汁淤积性肝病[例(%)] 1(2.1) 5(3.8) 自身免疫性肝病[例(%)] 1(2.1) 7(5.3) 隐源性肝病[例(%)] 9(19.1) 9(6.9) AST(U/L) 38(25~53) 31(23~41) Z=-2.712 0.007 ALT(U/L) 27(17~37) 22(16~31) Z=-1.272 0.203 Alb(g/L) 35.3±5.1 33.9±5.8 t=1.456 0.510 TBil(μmol/L) 19.50(14.60~33.80) 20.03(14.30~26.00) Z=-0.921 0.357 Cr(μmol/L) 61.00(53.60~72.40) 62. 50(52.80~73.22) Z=-0.290 0.772 PT(s) 14.05(13.30~14.90) 14.30(13.20~15.50) Z=-1.010 0.312 Hb(g/L) 102(80~111) 84(69~97) Z=-3.769 <0.001 PLT(×109/L) 80.5(69.0~144.0) 75.5(49.0~109.0) Z=-1.511 0.131 Child-Pugh评分(分) 6(5~7) 6(6~7) Z=-1.900 0.058 Child-Pugh A/B/C级(例) 31/16/0 72/55/4 χ2=2.705 0.259 HVPG(mmHg) 14.13±4.86 14.59±4.30 t=-0.611 0.408 注:AST,谷草转氨酶;ALT,谷丙转氨酶;Alb,白蛋白;TBil,总胆红素;Cr,肌酐;PT,凝血酶原时间;Hb,血红蛋白;PLT,血小板计数。
表 2 两组患者再出血的单因素分析
Table 2. Univariate analysis of liver cirrhosis rebleeding
项目 β值 HR 95%CI P值 年龄 0.016 1.016 0.996~1.037 0.107 HVPG -0.008 0.992 0.933~1.055 0.805 AST 0.003 1.003 0.955~1.012 0.466 ALT -0.002 0.998 0.987~1.008 0.679 Alb -0.003 0.968 0.926~1.012 0.151 TBil -0.007 0.993 0.973~1.014 0.533 Cr 0.004 1.004 1.001~1.007 0.019 PT 0.084 0.919 0.796~1.062 0.254 Hb -0.007 0.993 0.983~1.004 0.204 PLT 0.000 1.000 0.996~1.004 0.872 治疗方式 卡维地洛 vs 联合治疗 0.683 0.505 0.292~0.874 0.015 性别 男 vs 女 0.371 1.450 0.857~2.451 0.166 病因 乙型肝炎 vs 丙型肝炎 -1.016 0.362 0.162~0.810 0.013 乙型肝炎 vs 酒精 -1.245 0.288 0.074~1.119 0.072 乙型肝炎 vs 胆汁淤积性肝病 -0.542 0.582 0.239~1.415 0.232 乙型肝炎 vs 自身免疫性肝病 1.237 3.445 1.114~10.656 0.032 乙型肝炎 vs 隐源性肝病 -1.417 0.242 0.030~1.939 0.182 Child-Pugh分级 A级 vs B级 0.233 1.262 0.756~2.106 0.373 A级 vs C级 -11.957 0.970 脾切除或脾栓塞 无 vs 有 -0.266 0.767 0.346~1.698 0.513 肝性脑病 无 vs 有 0.004 1.004 0.136~7.414 0.997 腹水 无 vs 有 0.209 1.232 0.737~2.061 0.427 表 3 两组患者肝脏相关死亡的单因素分析
Table 3. Univariate analysis of liver related death
项目 β值 HR 95%CI P值 年龄 0.024 1.025 1.000~1.050 0.054 HVPG 0.062 1.064 0.991~1.141 0.087 AST 0.009 1.009 1.001~1.017 0.023 ALT 0.003 1.003 0.994~1.012 0.549 Alb -0.030 0.971 0.920~1.025 0.281 TBil 0.015 1.016 1.002~1.029 0.024 Cr 0.005 1.005 1.001~1.008 0.004 PT -0.056 0.945 0.789~1.133 0.541 Hb 0.001 1.001 0.989~1.014 0.862 PLT 0.000 1.000 0.995~1.005 0.985 治疗方式 卡维地洛 vs 联合治疗 -0.224 0.799 0.405~1.578 0.518 性别 男 vs 女 0.130 1.138 0.601~2.157 0.691 病因 乙型肝炎 vs 丙型肝炎 0.366 1.442 0.429~4.854 0.554 乙型肝炎 vs 酒精 0.555 1.742 0.871~3.483 0.116 乙型肝炎 vs 胆汁淤积性肝病 0.546 1.726 0.227~13.661 0.598 乙型肝炎 vs 自身免疫性肝病 -11.911 0.978 乙型肝炎 vs 隐源性肝病 0.163 1.177 0.348~3.984 0.793 Child-Pugh分级 A级 vs B级 0.609 1.839 0.991~3.408 0.053 A级 vs C级 -11.797 0.980 脾切除或脾栓塞 无 vs 有 -0.010 0.990 0.413~2.371 0.982 腹水 无 vs 有 0.257 1.293 0.696~2.402 0.415 肝性脑病 无 vs 有 -3.043 0.048 0.000~1 186.160 0.556 -
[1] VILLANUEVA C, ALBILLOS A, GENESCÀ J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension(PREDESCI): A randomised, double-blind, placebo-controlled, multicentre trial[J]. Lancet, 2019, 393( 10181): 1597- 1608. DOI: 10.1016/S0140-6736(18)31875-0. [2] de FRANCHIS R, BOSCH J, GARCIA-TSAO G, et al. Baveno VII- Renewing consensus in portal hypertension[J]. J Hepatol, 2022, 76( 4): 959- 974. DOI: 10.1016/j.jhep.2021.12.022. [3] WANG SN, WANG GC, ZHANG MY, et al. Prevention for rebleeding of esophageal varices under the guidance of hepatic venous pressure gradient[J/OL]. Chin J Dig Med Imageology Electron Ed, 2019, 9( 6): 256- 262. DOI: 10.3877/cma.j.issn.2095-2015.2019.06.005.王思宁, 王广川, 张明艳, 等. 肝静脉压力梯度指导下食管静脉曲张再出血预防方法选择[J/OL]. 中华消化病与影像杂志(电子版), 2019, 9( 6): 256- 262. DOI: 10.3877/cma.j.issn.2095-2015.2019.06.005. [4] REIBERGER T, ULBRICH G, FERLITSCH A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol[J]. Gut, 2013, 62( 11): 1634- 1641. DOI: 10.1136/gutjnl-2012-304038. [5] ALBILLOS A, KRAG A. Beta-blockers in the era of precision medicine in patients with cirrhosis[J]. J Hepatol, 2023, 78( 4): 866- 872. DOI: 10.1016/j.jhep.2022.12.005. [6] KIM SG, KIM TY, SOHN JH, et al. A randomized, multi-center, open-label study to evaluate the efficacy of carvedilol vs. propranolol to reduce portal pressure in patients with liver cirrhosis[J]. Am J Gastroenterol, 2016, 111( 11): 1582- 1590. DOI: 10.1038/ajg.2016.327. [7] BAO H, SUO RN. Progress in endoscopic treatment of acute esophageal variceal hemorrhage[J]. Int J Dig Dis, 2024, 44( 2): 71- 74. DOI: 10.3969/j.issn.1673-534X.2024.02.001.包涵, 索日娜. 急性食管静脉曲张破裂出血的内镜下治疗进展[J]. 国际消化病杂志, 2024, 44( 2): 71- 74. DOI: 10.3969/j.issn.1673-534X.2024.02.001. [8] CORTI MC, GAZIANO M, HENNEKENS CH. Iron status and risk of cardiovascular disease[J]. Ann Epidemiol, 1997, 7( 1): 62- 68. DOI: 10.1016/s1047-2797(96)00112-3. [9] MORALES CASTRO D, DRESSER L, GRANTON J, et al. Pharmacokinetic alterations associated with critical illness[J]. Clin Pharmacokinet, 2023, 62( 2): 209- 220. DOI: 10.1007/s40262-023-01213-x. [10] PHILIPS BJ, LANE KT, DIXON J, et al. The effects of acute renal failure on drug metabolism[J]. Expert Opin Drug Metab Toxicol, 2014, 10( 1): 11- 23. DOI: 10.1517/17425255.2013.835802. [11] ALMUKHTAR S. Transforming growth factor-β1 gene polymorphism rs1800471 and end-stage renal disease[J]. Br J Biomed Sci, 2021, 78( 4): 233- 235. DOI: 10.1080/09674845.2021.1908689. [12] LI L, WAN QS, YANG SK, et al. Impact of vitamin D receptor gene polymorphism on chronic renal failure susceptibility[J]. Ther Apher Dial, 2018, 22( 6): 575- 587. DOI: 10.1111/1744-9987.12714. [13] MUNGMUNPUNTIPANTIP R, WIWANITKIT V. PDGFRA gene polymorphism and high myopia: Correspondence[J]. Ophthalmic Genet, 2022, 43( 3): 430. DOI: 10.1080/13816810.2022.2068042. [14] WU ZY, WU ZG, QI HM, et al. Correlation between MRAS gene polymorphism and atherosclerosis[J]. Eur Rev Med Pharmacol Sci, 2020, 24( 10): 5644- 5649. DOI: 10.26355/eurrev_202005_21355. -



 PDF下载 ( 1700 KB)
PDF下载 ( 1700 KB)

 下载:
下载: