慢性乙型肝炎患者血清外泌体miR-122-5p表达水平与肝脏融合性坏死和纤维化转归的关系
DOI: 10.12449/JCH250514
Association of serum exosomal miR-122-5p with the prognosis of hepatic confluent necrosis and fibrosis in patients with chronic hepatitis B
-
摘要:
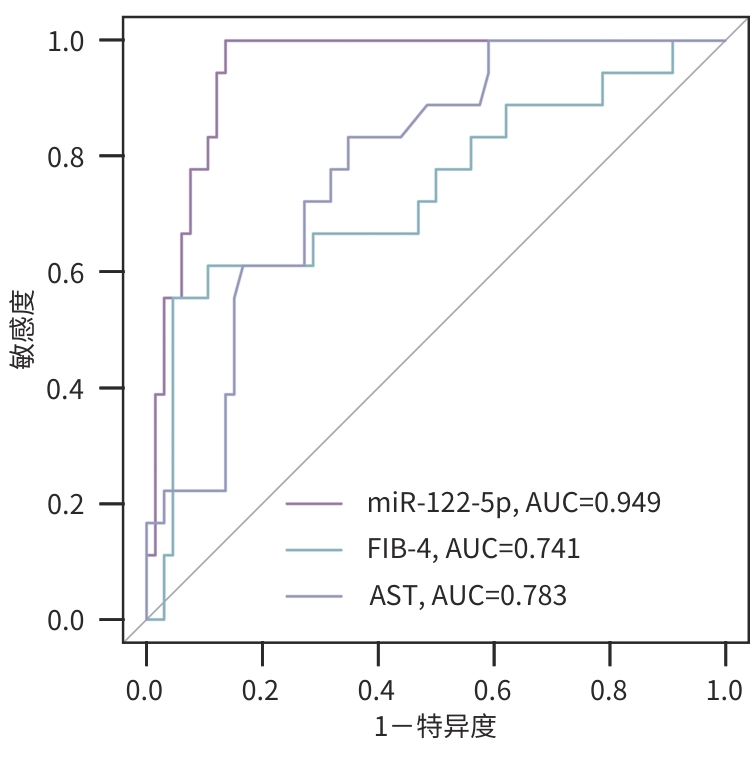
目的 探讨血清外泌体微RNA(miRNA)与慢性乙型肝炎(CHB)患者肝组织炎症损伤及组织学转归之间的关联。 方法 从6例健康者及6例CHB患者中采集外周血清,经尺寸洗脱色谱法提取外泌体。通过小RNA测序及转录组学分析,识别与肝组织炎症损伤和肝纤维化程度相关的血清外泌体miRNA,分别在脂多糖/D-氨基半乳糖诱导急性肝损伤小鼠模型、四氯化碳诱导肝纤维化大鼠模型及84例具有治疗前后两次肝活检病理评估的CHB患者中进行实时荧光定量PCR验证。正态分布的计量资料两组间比较采用成组t检验;多组间比较采用方差分析,进一步两两比较采用Tukey检验。非正态分布的计量资料两组间比较采用Mann-Whitney U检验;多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Dunn检验。计数资料组间比较采用χ2检验或Fisher精确检验。采用单因素和多因素Logistic回归分析影响因素。 结果 血清外泌体miR-122-5p在CHB患者中异常表达,并在伴有融合性坏死及晚期肝纤维化患者中表达均下调。在急性肝损伤小鼠模型和肝纤维化大鼠模型中,与对照组相比,模型组肝脏miR-122-5p的表达水平均显著降低(P值分别为0.048、0.014);与轻度肝损伤相比,伴有重度融合性坏死和晚期纤维化的肝组织中miR-122-5p的表达水平进一步显著下降(P值均<0.05)。在84例CHB患者中,伴肝组织重度融合性坏死或晚期肝纤维化患者,其血清外泌体miR-122-5p的表达显著低于轻度肝损伤患者(P值分别为<0.001、0.003)。多因素Logistic回归分析显示,miR-122-5p表达水平是融合性坏死(OR=0.001,95%CI:0.000~0.037,P=0.005)和肝纤维化程度(OR=0.568,95%CI:0.331~0.856,P=0.019)的独立影响因素。相较于miR-122-5p低表达患者,治疗前高水平表达患者在接受抗病毒治疗72周后其肝纤维化的逆转率更高(64.3% vs 38.1%,P=0.029)。 结论 CHB患者血清外泌体miR-122-5p与肝脏融合性坏死和纤维化进展密切相关,其表达水平降低可能加重肝脏融合性坏死,促进纤维化进展,并可能影响CHB患者接受抗病毒治疗后的肝组织学转归。 Abstract:Objective To investigate the association of serum exosomal microRNAs (miRNAs) with hepatic inflammatory injury and histological outcomes in patients with chronic hepatitis B (CHB). Methods Peripheral serum samples were collected from six healthy adults and six patients with CHB, and size exclusion chromatography was used to extract exosomes. Small RNA sequencing and transcriptomic analysis were used to identify the serum exosomal miRNAs associated with liver inflammatory injury and fibrosis, and quantitative real-time PCR was used for validation in a mouse model of acute liver injury induced by lipopolysaccharide/D-galactosamine, a rat model of liver fibrosis induced by carbon tetrachloride, and 84 CHB patients undergoing liver biopsy twice before and after treatment. The independent-samples t test was used for comparison of normally distributed continuous data between two groups; an analysis of variance was used for comparison between multiple groups, and the Tukey test was used for further comparison between two groups. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups; the Kruskal-Wallis H test was used for comparison between multiple groups, and the Dunn test was used for further comparison between two groups. The chi-square test or the Fisher’s exact test was used for comparison of categorical data between groups. The univariate and multivariate Logistic regression analyses were used to investigate influencing factors. Results Abnormal expression of serum exosomal miR-122-5p was observed in patients with CHB, and it was downregulated in patients with confluent necrosis and advanced fibrosis. In the mouse model of acute liver injury and the rat model of liver fibrosis, compared with the control group, the model group had a significant reduction in the expression level of miR-122-5p in the liver (P=0.048 and 0.014), and compared with the patients with mild liver injury, the patients with severe confluent necrosis and advanced fibrosis showed a significant reduction in the expression level of miR-122-5p in liver tissue (P<0.05). Among the 84 CHB patients, the patients with severe hepatic confluent necrosis or advanced liver fibrosis had a significantly lower expression level of serum exosomal miR-122-5p than those with mild liver injury (P<0.001 and P=0.003). The multivariate Logistic regression analysis showed that the expression level of miR-122-5p was an independent influencing factor for confluent necrosis (odds ratio [OR]=0.001, 95% confidence interval [CI]: 0.000 — 0.037, P=0.005) and liver fibrosis degree (OR=0.568, 95%CI: 0.331 — 0.856, P=0.019). In addition, compared with the patients with low expression of miR-122-5p, the patients with high expression of miR-122-5p before treatment had a significantly higher reversal rate of liver fibrosis after 72 weeks of antiviral therapy (64.3% vs 38.1%, P=0.029). Conclusion Serum exosomal miR-122-5p in CHB patients is closely associated with the progression of hepatic confluent necrosis and fibrosis, and the reduction in the expression level of miR-122-5p may aggravate hepatic confluent necrosis, promote the progression of fibrosis, and affect the histological outcome of CHB patients after antiviral therapy. -
Key words:
- Hepatitis B, Chronic /
- Hepatic Fibrosis /
- Necrosis /
- Exosomes /
- MicroRNAs
-
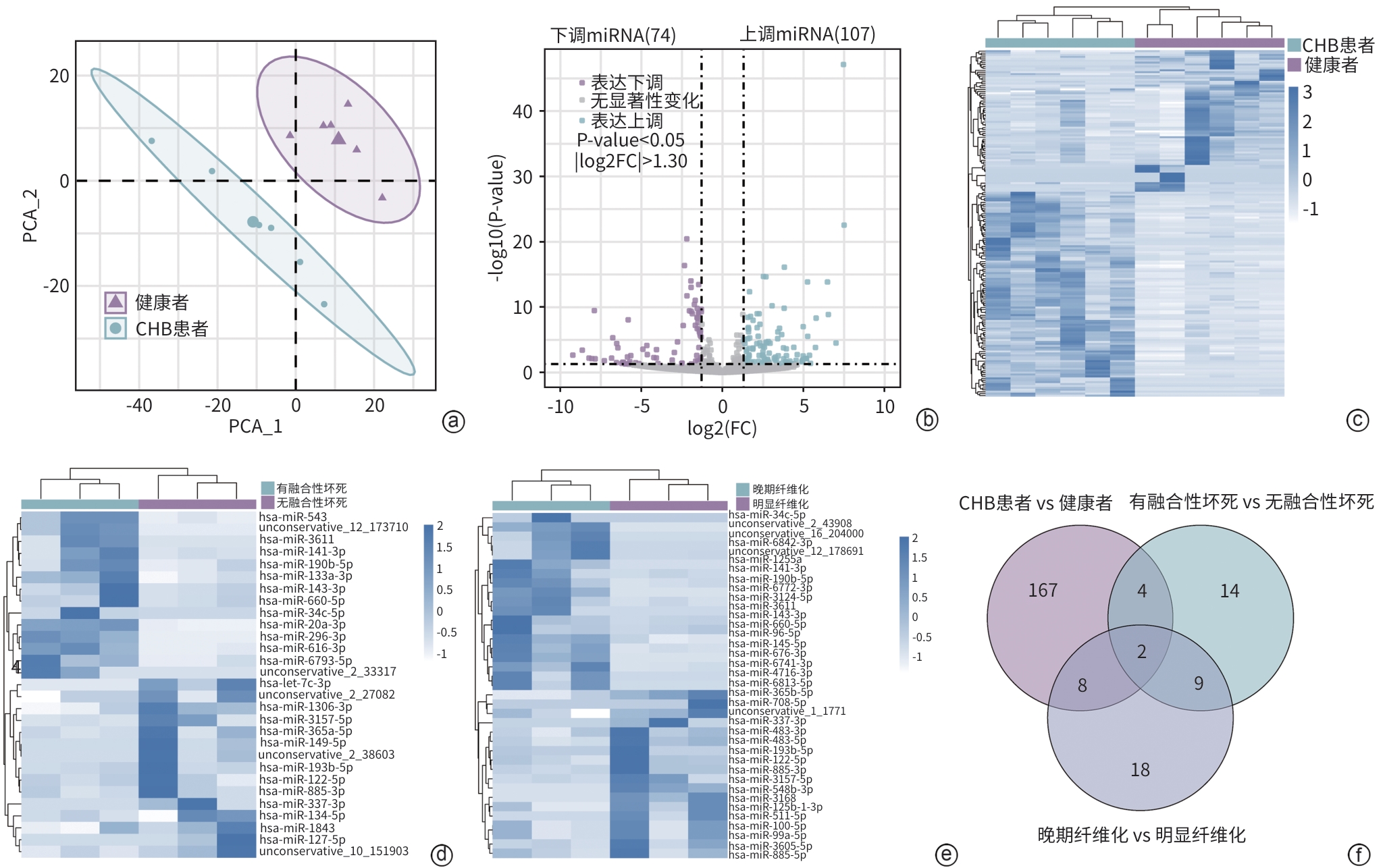
注: a,CHB患者与健康者miRNA谱的PCA结果;b,CHB患者与健康者的差异表达miRNA火山图;c,CHB患者与健康者的差异miRNA表达热图;d,有融合性坏死与无融合性坏死的CHB患者差异miRNA表达热图;e,晚期肝纤维化与明显肝纤维化的CHB患者差异miRNA表达热图;f,三组差异miRNA的韦恩图,共同交集为miR-122-5p和miR-885-3p。
图 1 CHB患者与健康者的血清外泌体miRNA转录组学分析
Figure 1. Transcriptomic analysis of serum exosomal miRNA in CHB patients and healthy adults
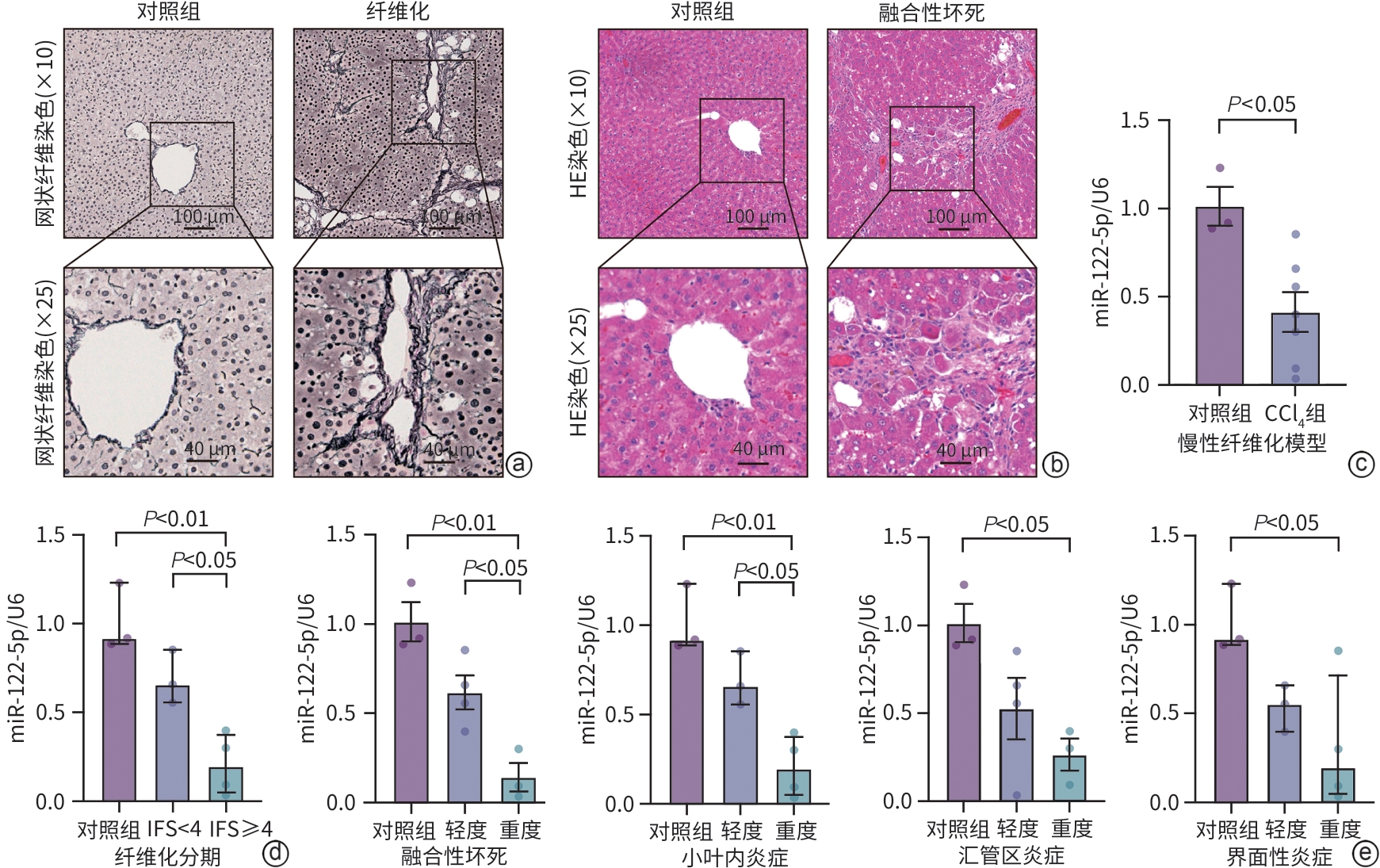
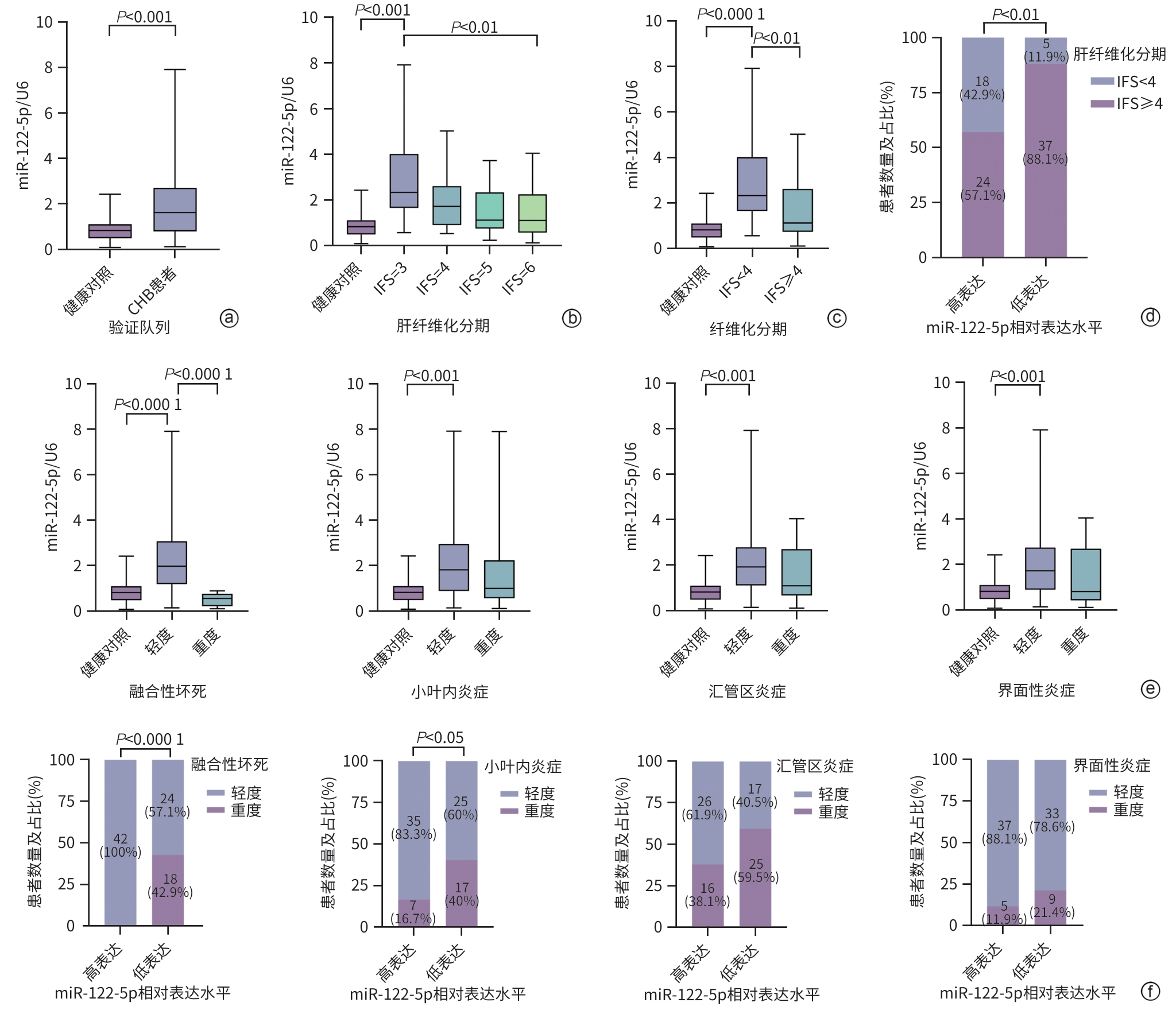
注: a,qRT-PCR检测健康者和CHB患者中miR-122-5p的表达;b,qRT-PCR检测miR-122-5p表达与不同肝纤维化分期的关系;c,qRT-PCR检测miR-122-5p在晚期肝纤维化和明显肝纤维化中的表达;d,miR-122-5p高、低表达组不同程度肝纤维化的患者比例;e,qRT-PCR检测miR-122-5p表达与四种肝脏炎症损伤严重程度的关系;f,miR-122-5p高、低表达组不同肝脏炎症损伤类型及程度的患者比例。
图 4 在CHB患者和健康者血清中的验证
Figure 4. Validation in the serum of CHB patients and healthy adults
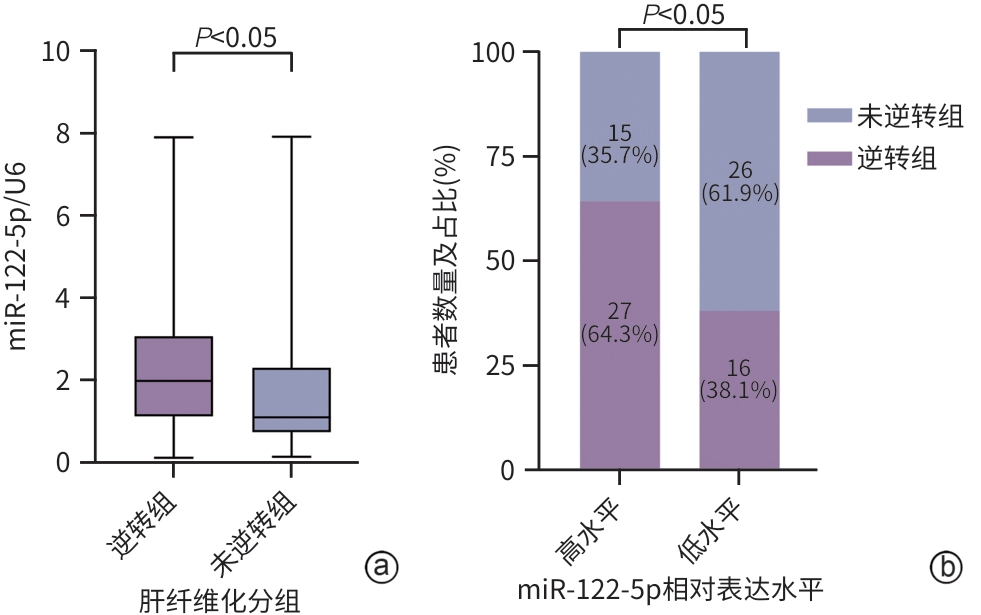
注: a,qRT-PCR比较miR-122-5p在肝纤维化逆转组和未逆转组患者中的表达;b,比较基线状态下miR-122-5p高、低表达水平的CHB患者在接受抗病毒治疗后组织学转归的差异。
图 7 CHB患者基线状态下血清外泌体miR-122-5p表达水平与治疗后肝组织学转归的关联性分析
Figure 7. Correlation analysis between serum exosomal miR-122-5p expression levels at baseline and histological outcomes of liver fibrosis after treatment in patients with CHB
表 1 发现队列的健康者和CHB患者的临床特征
Table 1. Clinical characteristics of healthy adults and CHB patients in the discovery cohort
指标 健康者(n=6) CHB患者(n=6) 年龄(岁) 41.50(37.00~48.50) 43.00(40.00~49.75) 男/女(例) 4/2 3/3 ALT(U/L) 18.80(10.00~26.00) 43.00(25.00~48.25) AST(U/L) 20.50(12.50~26.30) 33.00(30.25~38.00) TBil(μmol/L) 10.90(8.30~12.60) 13.05(11.47~14.10) Alb(g/L) 36.30(33.30~41.30) 44.50(42.50~47.25) PLT(×109/L) 190.70(152.50~246.80) 170.50(128.00~195.80) PT(s) 11.80(11.20~12.30) 10.55(10.50~11.28) HBsAg阳性(例) 0 6 HBeAg阳性(例) 0 0 HBV DNA(log10 IU/mL) 4.30(4.20~5.45) LSM(kPa) 11.60(9.60~11.95) APRI 0.50(0.43~0.65) FIB-4 1.60(1.13~2.23) 表 2 肝纤维化逆转组与未逆转组的CHB患者基线特征比较
Table 2. Comparison of baseline characteristics of CHB patients between the regressive and non-regressive group
指标 总计(n=84) 逆转组(n=43) 未逆转组(n=41) 统计值 P值 年龄(岁) 44.00±11.00 41.53±12.23 46.59±8.98 t=2.164 0.034 男[例(%)] 58(69.05) 30(69.77) 28(68.29) χ2=0.000 >0.05 饮酒史[例(%)] 19(22.62) 8(18.60) 11(26.83) χ2=0.409 0.522 吸烟史[例(%)] 20(23.81) 8(18.60) 12(29.27) χ2=0.793 0.373 BMI(kg/m2) 23.34±3.08 23.35±2.86 23.32±3.32 t=-0.053 0.958 ALT(U/L) 44.50(28.50~91.50) 44.00(25.50~78.00) 46.00(32.00~118.00) Z=-1.061 0.289 AST(U/L) 37.00(27.50~79.00) 34.00(24.50~65.00) 44.00(33.00~79.00) Z=-1.643 0.100 ALP(U/L) 83.00(67.00~110.50) 81.00(65.25~108.00) 92.00(74.00~111.00) Z=-0.948 0.343 GGT(U/L) 37.00(20.50~59.00) 25.00(18.00~53.00) 44.00(26.00~69.00) Z=-1.786 0.074 TBil(μmol/L) 13.90(10.96~20.35) 12.30(9.75~21.00) 14.80(11.20~19.80) Z=-1.105 0.269 Alb(g/L) 41.55±4.28 42.05±4.45 41.02±4.08 t=-1.106 0.272 PLT(×109/L) 160.63±54.63 180.93±50.42 139.34±51.13 t=-3.751 <0.001 LSM(kPa) 8.60(5.90~16.60) 7.40(5.55~9.20) 11.90(6.90~19.40) Z=-2.640 0.008 APRI 0.64(0.41~1.53) 0.47(0.29~1.23) 0.83(0.52~1.66) Z=-2.873 0.004 FIB-4 1.64(1.14~2.46) 1.41(1.01~2.03) 2.18(1.34~3.65) Z=-3.405 <0.001 HBeAg阳性[例(%)] 46(54.76) 25(58.14) 21(51.22) χ2=0.174 0.676 HBV DNA(log10 IU/mL) 6.09(4.50~7.42) 6.40(4.56~7.69) 5.83(4.47~7.26) Z=-0.609 0.543 界面性炎症[例(%)] χ2=0.000 >0.05 轻度 70(83.33) 36(83.72) 34(82.93) 重度 14(16.67) 7(16.28) 7(17.07) 融合性坏死[例(%)] χ2=2.085 0.149 轻度 66(78.57) 37(86.05) 29(70.73) 重度 18(21.43) 6(13.95) 12(29.27) 小叶内炎症[例(%)] χ2=1.145 0.285 轻度 60(71.43) 28(65.12) 32(78.05) 重度 24(28.57) 15(34.88) 9(21.95) 汇管区炎症[例(%)] χ2=2.320 0.128 轻度 43(51.19) 26(60.47) 17(41.46) 重度 41(48.81) 17(39.53) 24(58.54) mHAI[例(%)] 0.046 1~3分 9(10.71) 8(18.60) 1(2.44) 4~6分 33(39.29) 17(39.53) 16(39.02) 7~9分 32(38.10) 12(27.91) 20(48.78) ≥10分 10(11.90) 6(13.95) 4(9.76) IFS[例(%)] χ2=6.310 0.097 3分 23(27.38) 16(37.21) 7(17.07) 4分 11(13.10) 7(16.28) 4(9.76) 5分 17(20.24) 7(16.28) 10(24.39) 6分 33(39.29) 13(30.23) 20(48.78) 注:mHAI,改良组织学活动指数。
表 3 单因素Logistic回归分析
Table 3. Univariable Logistic regression analysis
项目 重度融合性坏死 晚期肝纤维化 OR(95%CI) P值 OR(95%CI) P值 BMI <23 kg/m2 Reference Reference ≥23 kg/m2 0.138(0.030~0.469) 0.004 1.833(0.698~5.012) 0.224 ALT 1.010(1.003~1.018) 0.011 1.007(0.999~1.019) 0.151 AST 1.015(1.006~1.027) 0.005 1.013(1.001~1.031) 0.078 ALP 1.013(1.000~1.028) 0.045 1.006(0.993~1.023) 0.377 GGT 1.010(1.003~1.020) 0.018 1.022(1.005~1.047) 0.033 PLT 0.986(0.975~0.996) 0.010 0.984(0.972~0.994) 0.005 LSM 1.084(1.028~1.152) 0.005 1.337(1.143~1.670) 0.003 APRI 1.827(1.233~2.972) 0.008 2.709(1.314~7.773) 0.026 FIB-4 1.381(1.108~1.806) 0.008 3.349(1.651~8.678) 0.005 miR-122-5p表达量 0.008(0.000~0.074) 0.001 0.565(0.379~0.776) 0.002 表 4 肝纤维化逆转的单因素和多因素Logistic回归分析
Table 4. Univariate and multivariate Logistic regression analysis of liver fibrosis reversal
项目 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄 0.957(0.916~0.996) 0.038 0.945(0.892~0.995) 0.038 PLT 1.017(1.007~1.028) 0.001 1.018(1.004~1.034) 0.016 LSM 0.949(0.896~0.997) 0.051 APRI 0.915(0.666~1.201) 0.529 FIB-4 0.743(0.545~0.941) 0.031 1.019(0.695~1.431) 0.917 mHAI 1~3分 Reference Reference 4~6分 0.133(0.007~0.843) 0.071 0.196(0.009~1.506) 0.170 7~9分 0.075(0.004~0.481) 0.021 0.169(0.008~1.399) 0.146 ≥10分 0.188(0.008~1.686) 0.178 0.588(0.019~10.042) 0.723 miR-122-5p相对表达水平 低水平 Reference Reference 高水平 2.925(1.222~7.250) 0.018 2.670(0.931~8.031) 0.072 -
[1] RONG GH, CHEN YP, YU ZJ, et al. Synergistic effect of Biejia-Ruangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: A multicenter, randomized, double-blind, placebo-controlled trial[J]. J Infect Dis, 2022, 225( 6): 1091- 1099. DOI: 10.1093/infdis/jiaa266. [2] MARCELLIN P, GANE E, BUTI M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study[J]. Lancet, 2013, 381( 9865): 468- 475. DOI: 10.1016/S0140-6736(12)61425-1. [3] ROCKEY DC, CALDWELL SH, GOODMAN ZD, et al. Liver biopsy[J]. Hepatology, 2009, 49( 3): 1017- 1044. DOI: 10.1002/hep.22742. [4] ISHAK K, BAPTISTA A, BIANCHI L, et al. Histological grading and staging of chronic hepatitis[J]. J Hepatol, 1995, 22( 6): 696- 699. DOI: 10.1016/0168-8278(95)80226-6. [5] JAY HL. Scheuer’s liver biopsy interpretation[M]. 9th ed. Singapore: Elsevier, 2016. [6] CHEN TJ, LIAW YF. The prognostic significance of bridging hepatic necrosis in chronic type B hepatitis: A histopathologic study[J]. Liver, 1988, 8( 1): 10- 16. DOI: 10.1111/j.1600-0676.1988.tb00960.x. [7] CHANG XJ, WANG J, CHEN Y, et al. A novel nomogram to predict evident histological liver injury in patients with HBeAg-positive chronic hepatitis B virus infection[J]. EBioMedicine, 2021, 67: 103389. DOI: 10.1016/j.ebiom.2021.103389. [8] ZHU L, YANG JR, YANG LL, et al. Advances on the application of transient elastography in the diagnosis of liver fibrosis[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 3): 16- 22. DOI: 10.3969/j.issn.1674-7380.2023.03.003.朱璐, 杨君茹, 何玲玲, 等. 瞬时弹性成像在肝纤维化诊断中的应用研究进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 3): 16- 22. DOI: 10.3969/j.issn.1674-7380.2023.03.003. [9] IMBERT-BISMUT F, RATZIU V, PIERONI L, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study[J]. Lancet, 2001, 357( 9262): 1069- 1075. DOI: 10.1016/S0140-6736(00)04258-6. [10] WILLIAMS AL, HOOFNAGLE JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis[J]. Gastroenterology, 1988, 95( 3): 734- 739. DOI: 10.1016/s0016-5085(88)80022-2. [11] WAI CT, GREENSON JK, FONTANA RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C[J]. Hepatology, 2003, 38( 2): 518- 526. DOI: 10.1053/jhep.2003.50346. [12] ADAMS LA, BULSARA M, ROSSI E, et al. Hepascore: An accurate validated predictor of liver fibrosis in chronic hepatitis C infection[J]. Clin Chem, 2005, 51( 10): 1867- 1873. DOI: 10.1373/clinchem.2005.048389. [13] STERLING RK, LISSEN E, CLUMECK N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection[J]. Hepatology, 2006, 43( 6): 1317- 1325. DOI: 10.1002/hep.21178. [14] CHEN Y, WANG YJ, CHEN YP, et al. A novel noninvasive program for staging liver fibrosis in untreated patients with chronic hepatitis B[J]. Clin Transl Gastroenterol, 2019, 10( 5): 1- 12. DOI: 10.14309/ctg.0000000000000033. [15] SHEN MY, SHEN Y, FAN XL, et al. Roles of macrophages and exosomes in liver diseases[J]. Front Med(Lausanne), 2020, 7: 583691. DOI: 10.3389/fmed.2020.583691. [16] PAN Y, TAN WF, YANG MQ, et al. The therapeutic potential of exosomes derived from different cell sources in liver diseases[J]. Am J Physiol Gastrointest Liver Physiol, 2022, 322( 4): G397- G404. DOI: 10.1152/ajpgi.00054.2021. [17] JIAO Y, LU W, XU P, et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure[J]. Hepatol Int, 2021, 15( 4): 957- 969. DOI: 10.1007/s12072-021-10217-3. [18] ZHAO TT, LI JF, ZHANG LT. Progress in the potential therapeutic mechanism of mesenchymal stem cell-derived exosomes for liver fibrosis[J]. Chin J Clin Pharmacol Ther, 2024, 29( 4): 475- 480. DOI: 10.12092/j.issn.1009-2501.2024.04.017.赵婷婷, 李俊峰, 张立婷. 间充质干细胞源性外泌体对肝纤维化潜在治疗机制的研究进展[J]. 中国临床药理学与治疗学, 2024, 29( 4): 475- 480. DOI: 10.12092/j.issn.1009-2501.2024.04.017. [19] NIU LJ, ZHANG YM, HUANG T, et al. Exosomal microRNA-155 as a biomarker for hepatic fibrosis diagnosis and progression[J]. Ann Transl Med, 2021, 9( 2): 137. DOI: 10.21037/atm-20-7787. [20] JI D, CHEN Y, BI JF, et al. Entecavir plus Biejia-Ruangan compound reduces the risk of hepatocellular carcinoma in Chinese patients with chronic hepatitis B[J]. J Hepatol, 2022, 77( 6): 1515- 1524. DOI: 10.1016/j.jhep.2022.07.018. [21] CHANG XJ, LV CH, WANG BQ, et al. The utility of P-I-R classification in predicting the on-treatment histological and clinical outcomes of patients with hepatitis B and advanced liver fibrosis[J]. Hepatology, 2024, 79( 2): 425- 437. DOI: 10.1097/HEP.0000000000000563. [22] BÖING AN, van der POL E, GROOTEMAAT AE, et al. Single-step isolation of extracellular vesicles by size-exclusion chromatography[J]. J Extracell Vesicles, 2014, 3: 23430. DOI: 10.3402/jev.v3.23430. [23] MATEESCU B, KOWAL EJ, van BALKOM BW, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA- an ISEV position paper[J]. J Extracell Vesicles, 2017, 6( 1): 1286095. DOI: 10.1080/20013078.2017.1286095. [24] LOVE MI, HUBER W, ANDERS S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2[J]. Genome Biol, 2014, 15( 12): 550. DOI: 10.1186/s13059-014-0550-8. [25] YANG WC, TAO KX, ZHANG P, et al. Maresin 1 protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting macrophage pyroptosis and inflammatory response[J]. Biochem Pharmacol, 2022, 195: 114863. DOI: 10.1016/j.bcp.2021.114863. [26] YANG Y, NEMOTO EM, HARVEY SK, et al. Increased hepatic platelet activating factor(PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis[J]. Gut, 2004, 53( 6): 877- 883. DOI: 10.1136/gut.2003.024893. [27] CHEN W, SUN YM, CHEN SY, et al. Matrisome gene-based subclassification of patients with liver fibrosis identifies clinical and molecular heterogeneities[J]. Hepatology, 2023, 78( 4): 1118- 1132. DOI: 10.1097/HEP.0000000000000423. [28] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Gastroenterology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Consensus on the diagnosis and therapy of hepatic fibrosis(2019)[J]. J Clin Hepatol, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. [29] SEKI E, SCHWABE RF. Hepatic inflammation and fibrosis: Functional links and key pathways[J]. Hepatology, 2015, 61( 3): 1066- 1079. DOI: 10.1002/hep.27332. [30] SUN XY, SU Y, WEE A, et al. Autoimmune hepatitis with confluent necrosis indicates severe liver injury but responds well to standard immunosuppressive therapy[J]. Histol Histopathol, 2024, 39( 7): 935- 945. DOI: 10.14670/HH-18-690. [31] PETRENKIENE V, GUDINAVICIENE I, JONAITIS L, et al. Improvement of liver histopathology in patients with hepatitis C after interferon and ribavirin combination therapy[J]. Medicina(Kaunas), 2004, 40( 10): 962- 968. [32] CHANG JH, NICOLAS E, MARKS D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1[J]. RNA Biol, 2004, 1( 2): 106- 113. DOI: 10.4161/rna.1.2.1066. [33] HSU SH, WANG B, KOTA J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver[J]. J Clin Invest, 2012, 122( 8): 2871- 2883. DOI: 10.1172/JCI63539. [34] LIU YN, CHEN WY, CHEN J, et al. miR-122-5p regulates hepatocytes damage caused by BaP and DBP co-exposure through SOCS1/STAT3 signaling in vitro[J]. Ecotoxicol Environ Saf, 2021, 223: 112570. DOI: 10.1016/j.ecoenv.2021.112570. [35] JIN Y, WONG YS, GOH BKP, et al. Circulating microRNAs as potential diagnostic and prognostic biomarkers in hepatocellular carcinoma[J]. Sci Rep, 2019, 9( 1): 10464. DOI: 10.1038/s41598-019-46872-8. [36] ZHANG YQ, ZHANG XZ, CHEN RF, et al. HSCs-derived exosomes regulate the levels of inflammatory cytokines in HIBECs through miR-122-5p mediated p38 MAPK signaling pathway[J]. Genomics, 2024, 116( 2): 110795. DOI: 10.1016/j.ygeno.2024.110795. [37] LI J, GHAZWANI M, ZHANG YF, et al. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression[J]. J Hepatol, 2013, 58( 3): 522- 528. DOI: 10.1016/j.jhep.2012.11.011. [38] TENG KY, BARAJAS JM, HU P, et al. Role of B cell lymphoma 2 in the regulation of liver fibrosis in miR-122 knockout mice[J]. Biology(Basel), 2020, 9( 7): 157. DOI: 10.3390/biology9070157. [39] WU ZY, WANG JB, FENG JY, et al. microRNA-122-5p prevents proliferation and promotes apoptosis of hepatic stellate cells by suppressing the cellular-Abelsongene/histone deacetylases 2 pathway[J]. Hum Exp Toxicol, 2022, 41: 9603271221084672. DOI: 10.1177/09603271221084672. [40] MIRZAEI HR, SAHEBKAR A, MOHAMMADI M, et al. Circulating microRNAs in hepatocellular carcinoma: Potential diagnostic and prognostic biomarkers[J]. Curr Pharm Des, 2016, 22( 34): 5257- 5269. DOI: 10.2174/1381612822666160303110838. [41] NAKAMURA M, KANDA T, JIANG X, et al. Serum microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2 binding protein are useful tools for liquid biopsy of the patients with hepatitis B virus and advanced liver fibrosis[J]. PLoS One, 2017, 12( 5): e0177302. DOI: 10.1371/journal.pone.0177302. -



 PDF下载 ( 4574 KB)
PDF下载 ( 4574 KB)

 下载:
下载: