微环境不同组分动态变化调控肝纤维化发展的特征与机制
DOI: 10.12449/JCH250423
The characteristics and mechanism of dynamic changes of different components in microenvironment in regulating the progression of liver fibrosis
-
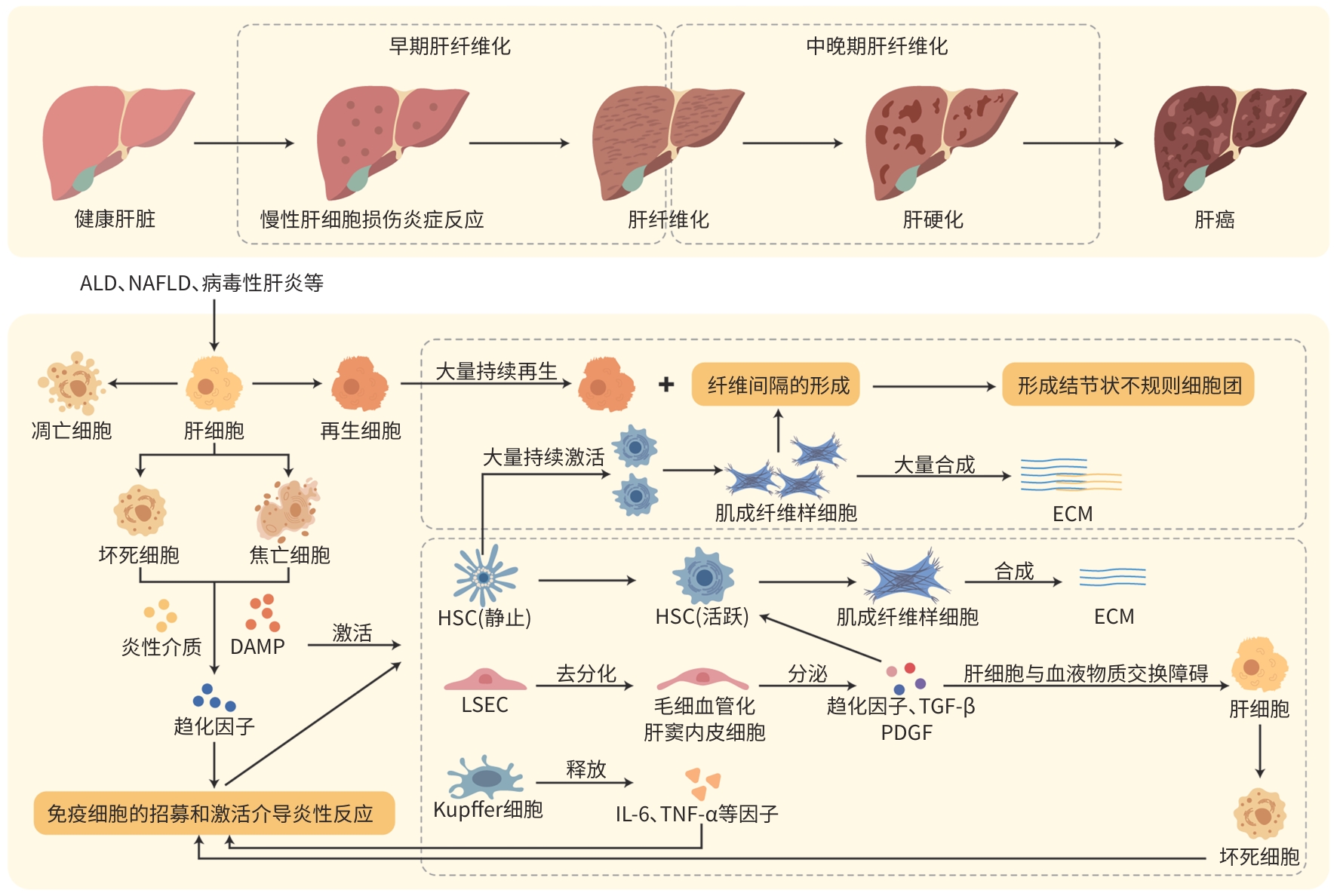
摘要: 肝脏有代谢、解毒、免疫防御等功能,肝脏微环境稳态的维持对机体健康极其重要。肝脏的微环境包括肝实质细胞、非实质细胞及非细胞成分等。不同病因导致肝脏慢性损伤性炎症反应,促进肝纤维化的形成和发展。在肝纤维化早、中晚不同时期的动态发展过程中,肝脏微环境的各种成分会发生一系列的变化,而这些能促进肝纤维化的恶性发展。深入探索微环境各成分变化机制对肝纤维化的发生机制和探索潜在的治疗策略至关重要。Abstract: The liver has diverse functions such as metabolism, detoxification, and immune defense, and the maintenance of hepatic microenvironment homeostasis is crucial for overall bodily health. The hepatic microenvironment consists of the components such as parenchymal cells, non-parenchymal cells, and non-cellular components. Chronic inflammatory responses induced by various etiological factors may promote the formation and progression of liver fibrosis. During the dynamic progression of liver fibrosis, from the early to advanced stages, various components within the hepatic microenvironment undergo a series of changes, which can promote the malignant progression of liver fibrosis. An in-depth exploration of the mechanisms underlying such changes in each component of the liver fibrosis microenvironment is of great significance for understanding the pathogenesis of liver fibrosis and discovering potential treatment strategies.
-
表 1 肝纤维化不同时期肝脏微环境各成分变化对比
Table 1. Comparison of changes in components of the liver microenvironment across different stages of liver fibrosis
类别 微环境成分 肝纤维化早期 肝纤维化中晚期 肝实质细胞 肝细胞 肝细胞的损伤和死亡 肝细胞大量损伤并再生,聚集形成假小叶性细胞结节,压迫血管促进门静脉高压的发生 肝非实质细胞 HSC 活化并增殖,形态上仍保留一定的规则性 大量激活并向成纤维样细胞转化,形态不规则,数量增加形成纤维间隔 Kupffer细胞 活化并释放TNF-α和IL-1等促炎因子, 刺激HSC的激活 进一步活化,释放更多的促炎因子和趋化因子如PDGF和MCP-1,并与其他免疫细胞相互作用 LSEC LSEC毛细血管化,获得基底膜,引起LSEC 功能障碍及毛细血管形成 LSEC毛细血管化加重,内皮功能障碍,肝窦内皮及内皮下基底膜脱落,导致肝脏血流动力学异常 肝非细胞成分 ECM 胶原蛋白和纤连蛋白的大量合成,未形成 明显的纤维间隔和结节且分布较均匀 Ⅰ型胶原的合成和沉淀显著增加,纤维间隔和结节形成,肝脏结构紊乱,ECM重构过度,异常表达和不均匀分布 炎性介质 主要为TNF-α、IL-6及IL-1的水平上升,参 与炎症的启动与维持,主要表现为促进炎 症反应 除TNF-α、IL-6及IL-1的水平上升外,氧化应激产物如ROS也增加,导致铁死亡损伤肝细胞,同时对肝脏结构和功能的影响更为显著,表现为促进纤维化 生长因子和 趋化因子 主要为TGF-β1、PDGF和VEGF表达水平 的升高,参与炎症反应,发挥作用的趋化 因子主要是MCP-1 其他生长因子如CTGF开始发挥作用,中晚期阶段还表达SDF-1等趋化因子,促进纤维母细胞和免疫细胞的进一步迁移与浸润 注:MCP-1,单核细胞趋化蛋白-1;ROS,活性氧;TGF-β1,转化生长因子β1;VEGF,血管内皮生长因子;CTGF,结缔组织生长因子;SDF-1,基质细胞衍生因子-1。
-
[1] GADD VL, ALEKSIEVA N, FORBES SJ. Epithelial plasticity during liver injury and regeneration[J]. Cell Stem Cell, 2020, 27( 4): 557- 573. DOI: 10.1016/j.stem.2020.08.016. [2] DRISKILL JH, PAN DJ. The hippo pathway in liver homeostasis and pathophysiology[J]. Annu Rev Pathol, 2021, 16: 299- 322. DOI: 10.1146/annurev-pathol-030420-105050. [3] BATALLER R, BRENNER DA. Liver fibrosis[J]. J Clin Invest, 2005, 115( 2): 209- 218. DOI: 10.1172/jci24282. [4] KUMAR S, DUAN QH, WU RX, et al. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis[J]. Adv Drug Deliv Rev, 2021, 176: 113869. DOI: 10.1016/j.addr.2021.113869. [5] WU BC, SODJI QH, OYELERE AK. Inflammation, fibrosis and cancer: Mechanisms, therapeutic options and challenges[J]. Cancers(Basel), 2022, 14( 3): 552. DOI: 10.3390/cancers14030552. [6] LI Y, LU LG, CAI XB. Liver regeneration and cell transplantation for end-stage liver disease[J]. Biomolecules, 2021, 11( 12): 1907. DOI: 10.3390/biom11121907. [7] DHAR D, BAGLIERI J, KISSELEVA T, et al. Mechanisms of liver fibrosis and its role in liver cancer[J]. Exp Biol Med(Maywood), 2020, 245( 2): 96- 108. DOI: 10.1177/1535370219898141. [8] HIGASHI T, FRIEDMAN SL, HOSHIDA Y. Hepatic stellate cells as key target in liver fibrosis[J]. Adv Drug Deliv Rev, 2017, 121: 27- 42. DOI: 10.1016/j.addr.2017.05.007. [9] MATSUDA M, SEKI E. Hepatic stellate cell-macrophage crosstalk in liver fibrosis and carcinogenesis[J]. Semin Liver Dis, 2020, 40( 3): 307- 320. DOI: 10.1055/s-0040-1708876. [10] de HAAN W, DHEEDENE W, APELT K, et al. Endothelial Zeb2 preserves the hepatic angioarchitecture and protects against liver fibrosis[J]. Cardiovasc Res, 2022, 118( 5): 1262- 1275. DOI: 10.1093/cvr/cvab148. [11] ROEHLEN N, CROUCHET E, BAUMERT TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives[J]. Cells, 2020, 9( 4): 875. DOI: 10.3390/cells9040875. [12] MURAO A, AZIZ M, WANG HC, et al. Release mechanisms of major DAMPs[J]. Apoptosis, 2021, 26( 3-4): 152- 162. DOI: 10.1007/s10495-021-01663-3. [13] SHI JJ, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526( 7575): 660- 665. DOI: 10.1038/nature15514. [14] HE S, TANG SL. WNT/β-catenin signaling in the development of liver cancers[J]. Biomed Pharmacother, 2020, 132: 110851. DOI: 10.1016/j.biopha.2020.110851. [15] MACDONALD BT, TAMAI K, HE X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases[J]. Dev Cell, 2009, 17( 1): 9- 26. DOI: 10.1016/j.devcel.2009.06.016. [16] TESTERINK N, AJAT M, HOUWELING M, et al. Replacement of retinyl esters by polyunsaturated triacylglycerol species in lipid droplets of hepatic stellate cells during activation[J]. PLoS One, 2012, 7( 4): e34945. DOI: 10.1371/journal.pone.0034945. [17] HERNANDEZ-GEA V, FRIEDMAN SL. Pathogenesis of liver fibrosis[J]. Annu Rev Pathol, 2011, 6: 425- 456. DOI: 10.1146/annurev-pathol-011110-130246. [18] MOUNTFORD S, EFFENBERGER M, NOLL-PUCHTA H, et al. Modulation of Liver Inflammation and Fibrosis by Interleukin-37[J]. Front Immunol, 2021, 12: 603649. DOI: 10.3389/fimmu.2021.603649. [19] YU YS, LIU Y, AN WS, et al. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis[J]. J Clin Invest, 2019, 129( 2): 546- 555. DOI: 10.1172/JCI121842. [20] FILLIOL A, PIQUET-PELLORCE C, RAGUÉNÈS-NICOL C, et al. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis[J]. J Hepatol, 2017, 66( 6): 1205- 1213. DOI: 10.1016/j.jhep.2017.01.005. [21] MAY D, DJONOV V, ZAMIR G, et al. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations[J]. PLoS One, 2011, 6( 7): e21478. DOI: 10.1371/journal.pone.0021478. [22] FABREGAT I, CABALLERO-DÍAZ D. Transforming growth factor-β- induced cell plasticity in liver fibrosis and hepatocarcinogenesis[J]. Front Oncol, 2018, 8: 357. DOI: 10.3389/fonc.2018.00357. [23] RODRÍGUEZ-RODRÍGUEZ DR, LOZANO-SEPULVEDA SA, DELGADO-MONTEMAYOR C, et al. Turnera diffusa extract attenuates profibrotic, extracellular matrix and mitochondrial markers in activated human hepatic stellate cells(HSC)[J]. Ann Hepatol, 2021, 22: 100281. DOI: 10.1016/j.aohep.2020.10.009. [24] YANG CM, YANG CC, HSU WH, et al. Tumor necrosis factor-α-induced C-C motif chemokine ligand 20 expression through TNF receptor 1-dependent activation of EGFR/p38 MAPK and JNK1/2/FoxO1 or the NF-κB pathway in human cardiac fibroblasts[J]. Int J Mol Sci, 2022, 23( 16): 9086. DOI: 10.3390/ijms23169086. [25] YAMASHITA M, PASSEGUÉ E. TNF-α coordinates hematopoietic stem cell survival and myeloid regeneration[J]. Cell Stem Cell, 2019, 25( 3): 357- 372. e 7. DOI: 10.1016/j.stem.2019.05.019. [26] SCHMIDT-ARRAS D, ROSE-JOHN S. IL-6 pathway in the liver: From physiopathology to therapy[J]. J Hepatol, 2016, 64( 6): 1403- 1415. DOI: 10.1016/j.jhep.2016.02.004. [27] YOUSEFI A, NAJAFI M, MOTAMED F, et al. Association of interleukin-6 and interleukin-1 family gene polymorphisms in autoimmune hepatitis[J]. Ann Hepatol, 2018, 17( 6): 1021- 1025. DOI: 10.5604/01.3001.0012.7202. [28] KARLMARK KR, WEISKIRCHEN R, ZIMMERMANN HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis[J]. Hepatology, 2009, 50( 1): 261- 274. DOI: 10.1002/hep.22950. [29] KONG DS, ZHANG ZL, CHEN LP, et al. Curcumin blunts epithelial-mesenchymal transition of hepatocytes to alleviate hepatic fibrosis through regulating oxidative stress and autophagy[J]. Redox Biol, 2020, 36: 101600. DOI: 10.1016/j.redox.2020.101600. [30] YING HZ, CHEN Q, ZHANG WY, et al. PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics(Review)[J]. Mol Med Rep, 2017, 16( 6): 7879- 7889. DOI: 10.3892/mmr.2017.7641. [31] WISZNIAK S, SCHWARZ Q. Exploring the intracrine functions of VEGF-A[J]. Biomolecules, 2021, 11( 1): 128. DOI: 10.3390/biom11010128. [32] ZHOU WC, ZHANG QB, QIAO L. Pathogenesis of liver cirrhosis[J]. World J Gastroenterol, 2014, 20( 23): 7312- 7324. DOI: 10.3748/wjg.v20.i23.7312. [33] IWAKIRI Y. Pathophysiology of portal hypertension[J]. Clin Liver Dis, 2014, 18( 2): 281- 291. DOI: 10.1016/j.cld.2013.12.001. [34] GRESSNER AM, WEISKIRCHEN R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets[J]. J Cell Mol Med, 2006, 10( 1): 76- 99. DOI: 10.1111/j.1582-4934.2006.tb00292.x. [35] WOHLLEBER D, KNOLLE PA. The role of liver sinusoidal cells in local hepatic immune surveillance[J]. Clin Transl Immunology, 2016, 5( 12): e117. DOI: 10.1038/cti.2016.74. [36] JEONG WI, PARK O, RADAEVA S, et al. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity[J]. Hepatology, 2006, 44( 6): 1441- 1451. DOI: 10.1002/hep.21419. [37] MCCONNELL MJ, KOSTALLARI E, IBRAHIM SH, et al. The evolving role of liver sinusoidal endothelial cells in liver health and disease[J]. Hepatology, 2023, 78( 2): 649- 669. DOI: 10.1097/HEP.0000000000000207. [38] MARTIN IV, BORKHAM-KAMPHORST E, ZOK S, et al. Platelet-derived growth factor(PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis[J]. Am J Pathol, 2013, 182( 1): 107- 117. DOI: 10.1016/j.ajpath.2012.09.006. [39] MENG Y, ZHAO T, ZHANG ZY, et al. The role of hepatic microenvironment in hepatic fibrosis development[J]. Ann Med, 2022, 54( 1): 2830- 2844. DOI: 10.1080/07853890.2022.2132418. [40] DU WJ, ZHEN JH, ZENG ZQ, et al. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection: IL-17 in HBV infection[J]. Diagn Pathol, 2013, 8: 40. DOI: 10.1186/1746-1596-8-40. [41] YANG WC, WANG YX, ZHANG CG, et al. Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation[J]. Front Pharmacol, 2022, 13: 865689. DOI: 10.3389/fphar.2022.865689. [42] YUAN SY, WEI C, LIU GF, et al. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway[J]. Cell Prolif, 2022, 55( 1): e13158. DOI: 10.1111/cpr.13158. [43] NAGARAJA T, CHEN L, BALASUBRAMANIAN A, et al. Correction: Activation of the connective tissue growth factor(CTGF)-transforming growth factor β1(TGF-β1) axis in hepatitis C virus-expressing hepatocytes[J]. PLoS One, 2023, 18( 8): e0290786. DOI: 10.1371/journal.pone.0290786. [44] LIEPELT A, TACKE F. Stromal cell-derived factor-1(SDF-1) as a target in liver diseases[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 311( 2): G203- G209. DOI: 10.1152/ajpgi.00193.2016. -



 PDF下载 ( 1177 KB)
PDF下载 ( 1177 KB)

 下载:
下载:


