基于女性偏倚现象探讨性激素调控T淋巴细胞介导自身免疫性肝炎的作用机制
DOI: 10.12449/JCH250421
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:王海强、孙大沙负责文章的构思与设计,论文撰写;王晗、田家华负责论文修订;崔馨月负责论文修订与审校;李明负责最终版本修订。
Mechanism of action of sex hormones in regulating T cell-mediated autoimmune hepatitis: A study based on the phenomenon of female bias
-
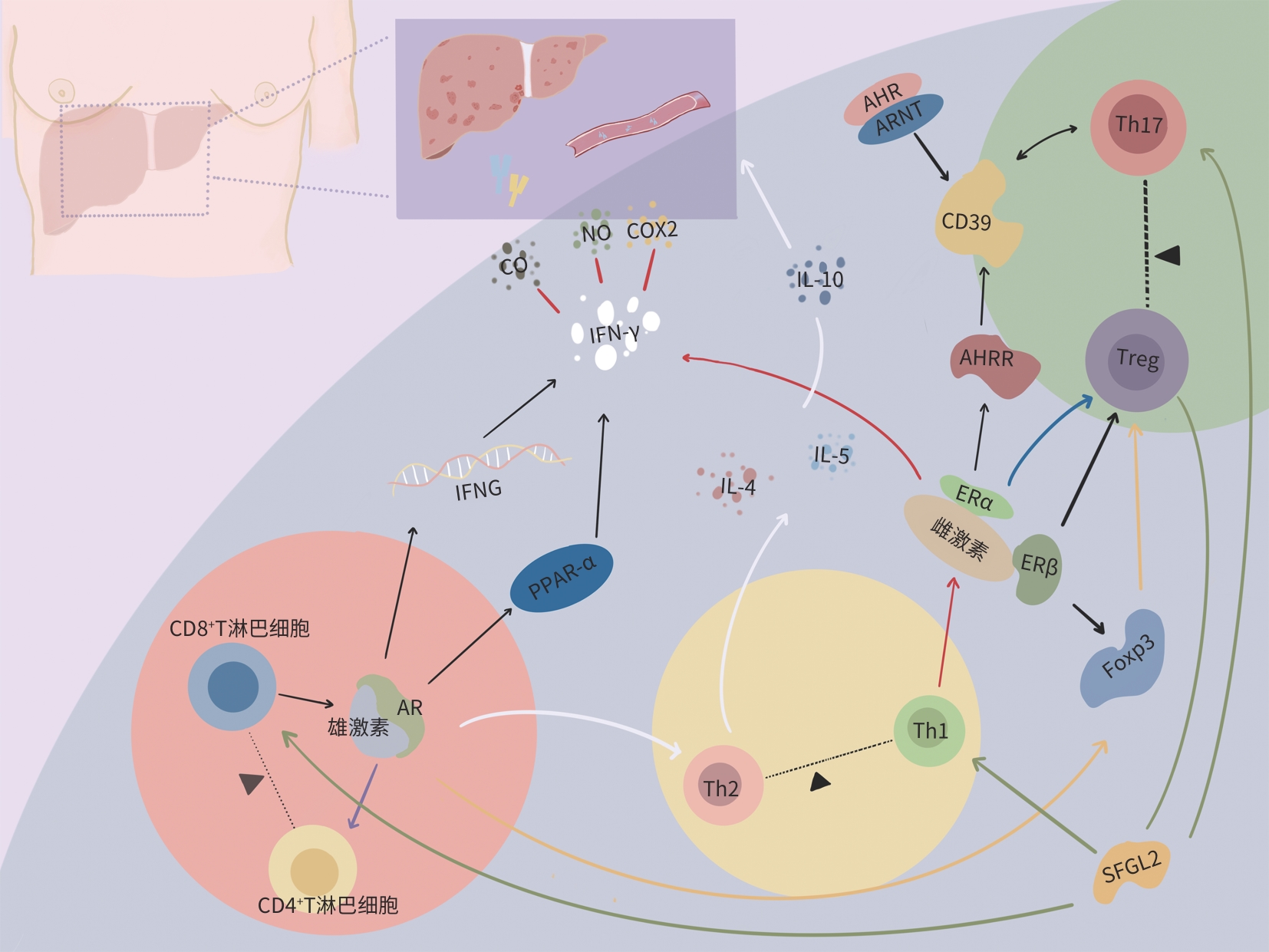
摘要: 自身免疫性肝炎(AIH)是一种以肝实质破坏和慢性纤维化为特征的自身免疫性疾病,常由T淋巴细胞介导发生。AIH的发病机制涉及多个因素,包括性别、地域、环境因素以及遗传易感性等。在AIH中,常出现女性偏倚现象,即女性发病风险远高于男性,这种性别差异与多种因素相关,性激素可能是导致AIH女性偏向性的重要因素之一,然而机制尚不完全清楚。深入了解性激素在AIH发病中的作用机制将有助于更好理解该病的发病机理,并可能为未来的治疗方法及预防策略的开发提供重要线索。本文综述雌激素和雄激素通过调控T淋巴细胞调节AIH发病的作用机制,以期为深入探讨性激素在AIH病因学中的潜在作用提供新思路和新方向。Abstract: Autoimmune hepatitis (AIH) is an autoimmune disease characterized by liver parenchymal destruction and chronic fibrosis, and it is often mediated by T cells. The pathogenesis of AIH involves multiple factors, including sex, region, environmental factors, and genetic susceptibility. A notable predisposition is observed in female individuals, and the incidence rate of AIH in female individuals is significantly higher than that in male individuals. This sex difference is associated with various factors, and sex hormones may be an important cause of the female predominance of AIH, although the specific mechanisms remain unclear. An in-depth understanding of the mechanism of action of sex hormones in the pathogenesis of AIH will help to better understand the pathogenesis of the disease and may provide important clues for developing future treatment methods and prevention strategies. This article reviews the mechanism of action of estrogen and androgen in regulating the pathogenesis of AIH by regulating T cells, in order to provide new ideas and directions for further exploring the potential role of sex hormones in the etiology of autoimmune diseases.
-
Key words:
- Hepatitis, Autoimmune /
- Bias /
- Gonadal Steroid Hormones /
- T-Lymphocytes
-
[1] MURATORI L, LOHSE AW, LENZI M. Diagnosis and management of autoimmune hepatitis[J]. BMJ, 2023, 380: e070201. DOI: 10.1136/bmj-2022-070201. [2] HAN B, TU CT. Diagnosis and management of autoimmune hepatitis in specific clinical phenotypes and patient populations: Current concepts[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 4): 16- 21.韩冰, 涂传涛. 特殊临床表型及特殊人群自身免疫性肝炎诊治现状[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 4): 16- 21. [3] FORSYTH KS, JIWRAJKA N, LOVELL CD, et al. The conneXion between sex and immune responses[J]. Nat Rev Immunol, 2024, 24( 7): 487- 502. DOI: 10.1038/s41577-024-00996-9. [4] MA X, WANG Q, XIAO X, et al. Guidelines on the diagnosis and management of autoimmune hepatitis(2021)[J]. J Clin Hepatol, 2022, 38( 1): 42- 49. DOI: 10.3760/cma.j.cn112138-20211112-00796.马雄, 王绮夏, 肖潇, 等. 自身免疫性肝炎诊断和治疗指南(2021)[J]. 临床肝胆病杂志, 2022, 38( 1): 42- 49. DOI: 10.3760/cma.j.cn112138-20211112-00796. [5] LAMBA M, NGU JH, STEDMAN CAM. Trends in incidence of autoimmune liver diseases and increasing incidence of autoimmune hepatitis[J]. Clin Gastroenterol Hepatol, 2021, 19( 3): 573- 579. e 1. DOI: 10.1016/j.cgh.2020.05.061. [6] TRIVEDI PJ, HIRSCHFIELD GM. Recent advances in clinical practice: Epidemiology of autoimmune liver diseases[J]. Gut, 2021, 70( 10): 1989- 2003. DOI: 10.1136/gutjnl-2020-322362. [7] TRIVEDI PJ, HIRSCHFIELD GM, ADAMS DH, et al. Immunopathogenesis of primary biliary cholangitis, primary sclerosing cholangitis and autoimmune hepatitis: Themes and concepts[J]. Gastroenterology, 2024, 166( 6): 995- 1019. DOI: 10.1053/j.gastro.2024.01.049. [8] COLAPIETRO F, MAISONNEUVE P, LYTVYAK E, et al. Incidence and predictors of hepatocellular carcinoma in patients with autoimmune hepatitis[J]. J Hepatol, 2024, 80( 1): 53- 61. DOI: 10.1016/j.jhep.2023.09.010. [9] LIBERT C, DEJAGER L, PINHEIRO I. The X chromosome in immune functions: When a chromosome makes the difference[J]. Nat Rev Immunol, 2010, 10( 8): 594- 604. DOI: 10.1038/nri2815. [10] HEYMANN F, TACKE F. Immunology in the liver: From homeostasis to disease[J]. Nat Rev Gastroenterol Hepatol, 2016, 13( 2): 88- 110. DOI: 10.1038/nrgastro.2015.200. [11] VUERICH M, WANG N, KALBASI A, et al. Dysfunctional immune regulation in autoimmune hepatitis: From pathogenesis to novel therapies[J]. Front Immunol, 2021, 12: 746436. DOI: 10.3389/fimmu.2021.746436. [12] STARK JM, TIBBITT CA, COQUET JM. The metabolic requirements of Th2 cell differentiation[J]. Front Immunol, 2019, 10: 2318. DOI: 10.3389/fimmu.2019.02318. [13] CHEN HR, HAN ZY, FAN YY, et al. CD4+ T-cell subsets in autoimmune hepatitis: A review[J]. Hepatol Commun, 2023, 7( 10): e0269. DOI: 10.1097/HC9.0000000000000269. [14] HENEGHAN MA, YEOMAN AD, VERMA S, et al. Autoimmune hepatitis[J]. Lancet, 2013, 382( 9902): 1433- 1444. DOI: 10.1016/S0140-6736(12)62163-1. [15] TAUBERT R, HARDTKE-WOLENSKI M, NOYAN F, et al. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies[J]. J Hepatol, 2014, 61( 5): 1106- 1114. DOI: 10.1016/j.jhep.2014.05.034. [16] BEHFARJAM F, SANATI MH, NASSERI MOGHADDAM S, et al. Role of Th1/Th2 cells and related cytokines in autoimmune hepatitis[J]. Turk J Gastroenterol, 2017, 28( 2): 110- 114. DOI: 10.5152/tjg.2017.17501. [17] AKKOÇ T. Re: The role of Th1/Th2 cells and associated cytokines in autoimmune hepatitis[J]. Turk J Gastroenterol, 2017, 28( 2): 115- 116. DOI: 10.5152/tjg.2017.14021. [18] ZHU JX, CHEN HX, CUI JJ, et al. Oroxylin A inhibited autoimmune hepatitis-induced liver injury and shifted Treg/Th17 balance to Treg differentiation[J]. Exp Anim, 2023, 72( 3): 367- 378. DOI: 10.1538/expanim.22-0171. [19] SONG JG, DAI J, CHEN XP, et al. Bifidobacterium mitigates autoimmune hepatitis by regulating IL-33-induced Treg/Th17 imbalance via the TLR2/4 signaling pathway[J]. Histol Histopathol, 2024, 39( 5): 623- 632. DOI: 10.14670/HH-18-669. [20] JI WW, PENG X, LOU TL, et al. Total flavonoids from Tetrastigma hemsleyanum ameliorates inflammatory stress in concanavalin A-induced autoimmune hepatitis mice by regulating Treg/Th17 immune homeostasis[J]. Inflammopharmacology, 2019, 27( 6): 1297- 1307. DOI: 10.1007/s10787-019-00599-0. [21] MA L, ZHANG LW, ZHUANG Y, et al. The imbalance between Foxp3+Tregs and Th1/Th17/Th22 cells in patients with newly diagnosed autoimmune hepatitis[J]. J Immunol Res, 2018, 2018: 3753081. DOI: 10.1155/2018/3753081. [22] AI G, YAN WM, YU HJ, et al. Soluble Fgl2 restricts autoimmune hepatitis progression via suppressing Tc17 and conventional CD8+ T cell function[J]. J Gene Med, 2018, 20( 7-8): e3023. DOI: 10.1002/jgm.3023. [23] MOULTON VR. Sex hormones in acquired immunity and autoimmune disease[J]. Front Immunol, 2018, 9: 2279. DOI: 10.3389/fimmu.2018.02279. [24] XING EZ, BILLI AC, GUDJONSSON JE. Sex bias and autoimmune diseases[J]. J Invest Dermatol, 2022, 142( 3 Pt B): 857- 866. DOI: 10.1016/j.jid.2021.06.008. [25] BROWN MA, SU MA. An inconvenient variable: Sex hormones and their impact on T cell responses[J]. J Immunol, 2019, 202( 7): 1927- 1933. DOI: 10.4049/jimmunol.1801403. [26] CHAKRABORTY B, BYEMERWA J, KREBS T, et al. Estrogen receptor signaling in the immune system[J]. Endocr Rev, 2023, 44( 1): 117- 141. DOI: 10.1210/endrev/bnac017. [27] THOMAS P, PANG Y, FILARDO EJ, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells[J]. Endocrinology, 2005, 146( 2): 624- 632. DOI: 10.1210/en.2004-1064. [28] SINGH RP, HAHN BH, BISCHOFF DS. Interferon genes are influenced by 17β-estradiol in SLE[J]. Front Immunol, 2021, 12: 725325. DOI: 10.3389/fimmu.2021.725325. [29] ADURTHI S, KUMAR MM, VINODKUMAR HS, et al. Oestrogen Receptor-α binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer[J]. Sci Rep, 2017, 7( 1): 17289. DOI: 10.1038/s41598-017-17102-w. [30] MOHAMMAD I, STARSKAIA I, NAGY T, et al. Estrogen receptor α contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation[J]. Sci Signal, 2018, 11( 526): eaap9415. DOI: 10.1126/scisignal.aap9415. [31] McNEER SK, KOCINSKI AD, GOODMAN WA. Murine CD4+ T cells exhibit sexually dimorphic responses to estrogen signaling[J]. J Immunol, 2023, 210( 1_Supplement): 64. 13. DOI: 10.4049/jimmunol.210.supp.64.13. [32] GOODMAN WA, BEDOYAN SM, HAVRAN HL, et al. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine[J]. Proc Natl Acad Sci USA, 2020, 117( 29): 17166- 17176. DOI: 10.1073/pnas.2002266117. [33] MCMURRAY RW, SUWANNAROJ S, NDEBELE K, et al. Differential effects of sex steroids on T and B cells: Modulation of cell cycle phase distribution, apoptosis and bcl-2 protein levels[J]. Pathobiology, 2001, 69( 1): 44- 58. DOI: 10.1159/000048757. [34] KWON H, SCHAFER JM, SONG NJ, et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer[J]. Sci Immunol, 2022, 7( 73): eabq2630. DOI: 10.1126/sciimmunol.abq2630. [35] HOFFMANN JP, LIU JA, SEDDU K, et al. Sex hormone signaling and regulation of immune function[J]. Immunity, 2023, 56( 11): 2472- 2491. DOI: 10.1016/j.immuni.2023.10.008. [36] WALECKI M, EISEL F, KLUG J, et al. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells[J]. Mol Biol Cell, 2015, 26( 15): 2845- 2857. DOI: 10.1091/mbc.E14-08-1323. [37] ZHU ML, BAKHRU P, CONLEY B, et al. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator[J]. Nat Commun, 2016, 7: 11350. DOI: 10.1038/ncomms11350. [38] LYU TT. Diethylstilbestrol inhibits acute autoimmune hepatitis induced by concanavalin A in mice[D]. Guangzhou: Southern Medical University, 2013.吕廷廷. 己烯雌酚抑制刀豆蛋白A诱导的小鼠急性自身免疫性肝炎[D]. 广州: 南方医科大学, 2013. [39] ZHANG YW, WANG L, QIU SL, et al. Effect of high concentration estradiol on acute autoimmune hepatitis induced by ConA[J]. J Sun Yat Sen Univ Med Sci, 2021, 42( 2): 185- 192. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2021.0025.张译文, 汪黎, 邱素苈, 等. 高浓度雌二醇对刀豆蛋白A诱导小鼠急性自身免疫性肝炎的影响[J]. 中山大学学报(医学科学版), 2021, 42( 2): 185- 192. DOI: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2021.0025. [40] LIBERAL R, GRANT CR, MA Y, et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease[J]. J Autoimmun, 2016, 72: 102- 112. DOI: 10.1016/j.jaut.2016.05.005. [41] VUERICH M, HARSHE R, FRANK LA, et al. Altered aryl-hydrocarbon-receptor signalling affects regulatory and effector cell immunity in autoimmune hepatitis[J]. J Hepatol, 2021, 74( 1): 48- 57. DOI: 10.1016/j.jhep.2020.06.044. [42] GUAN XN, POLESSO F, WANG CJ, et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy[J]. Nature, 2022, 606( 7915): 791- 796. DOI: 10.1038/s41586-022-04522-6. [43] ZHANG MA, AHN JJ, ZHAO FL, et al. Antagonizing peroxisome proliferator-activated receptor α activity selectively enhances Th1 immunity in male mice[J]. J Immunol, 2015, 195( 11): 5189- 5202. DOI: 10.4049/jimmunol.1500449. [44] BEN-BATALLA I, VARGAS-DELGADO ME, von AMSBERG G, et al. Influence of androgens on immunity to self and foreign: Effects on immunity and cancer[J]. Front Immunol, 2020, 11: 1184. DOI: 10.3389/fimmu.2020.01184. [45] MARTINELLI S, NANNINI G, CIANCHI F, et al. The impact of microbiota-immunity-hormone interactions on autoimmune diseases and infection[J]. Biomedicines, 2024, 12( 3): 616. DOI: 10.3390/biomedicines12030616. [46] SUN C, ZHU DZ, ZHU Q, et al. The significance of gut microbiota in the etiology of autoimmune hepatitis: A narrative review[J]. Front Cell Infect Microbiol, 2024, 14: 1337223. DOI: 10.3389/fcimb.2024.1337223. [47] CHENG ZL, YANG L, CHU HK. The gut microbiota: A novel player in autoimmune hepatitis[J]. Front Cell Infect Microbiol, 2022, 12: 947382. DOI: 10.3389/fcimb.2022.947382. -



 PDF下载 ( 1026 KB)
PDF下载 ( 1026 KB)

 下载:
下载:


