基于炎症因子、肺超声和CT评分系统的急性胰腺炎预后不良列线图预测模型的构建
DOI: 10.12449/JCH250417
Establishment of a nomogram prediction model for poor prognosis of acute pancreatitis based on inflammatory factors, lung ultrasound, and CT scores
-
摘要:
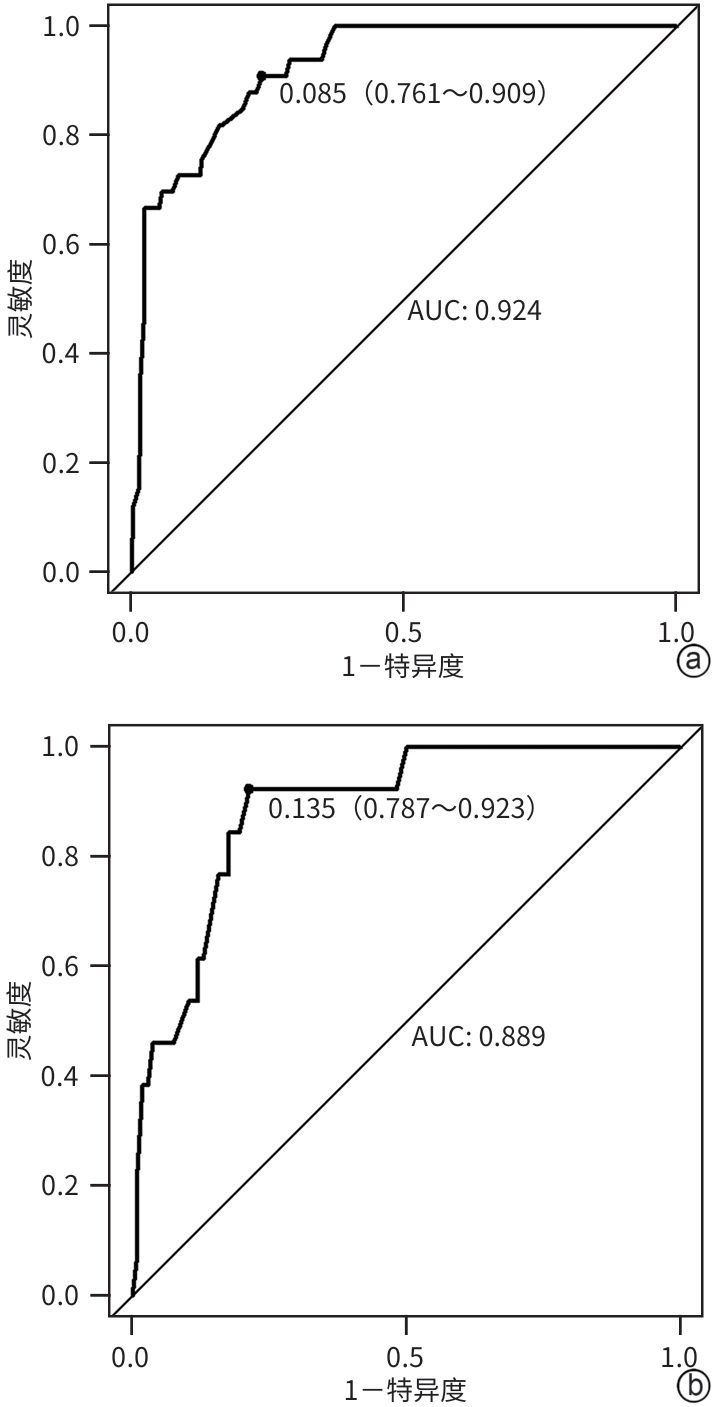
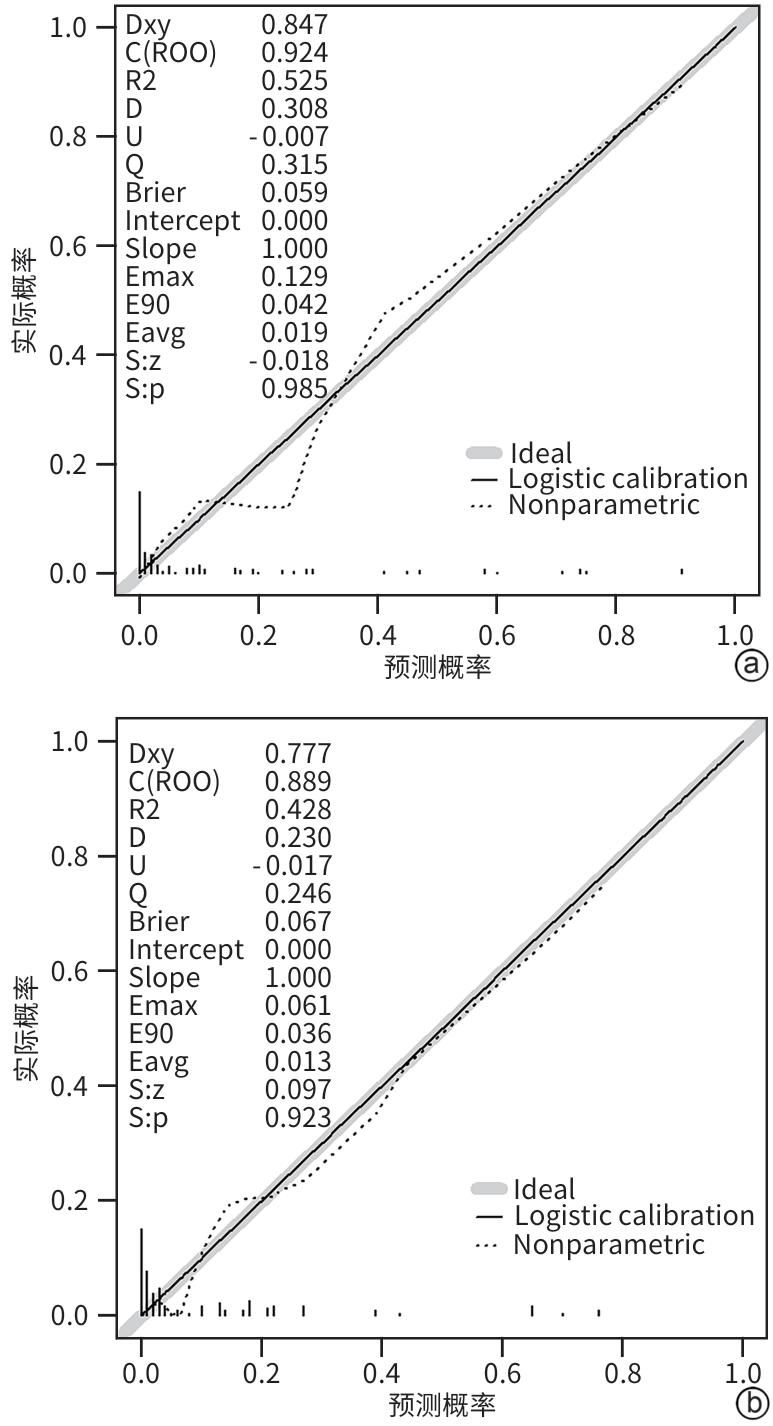
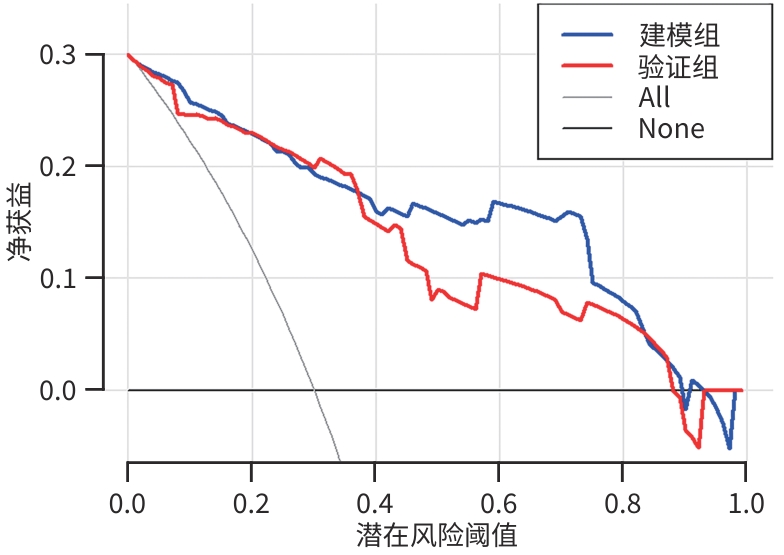
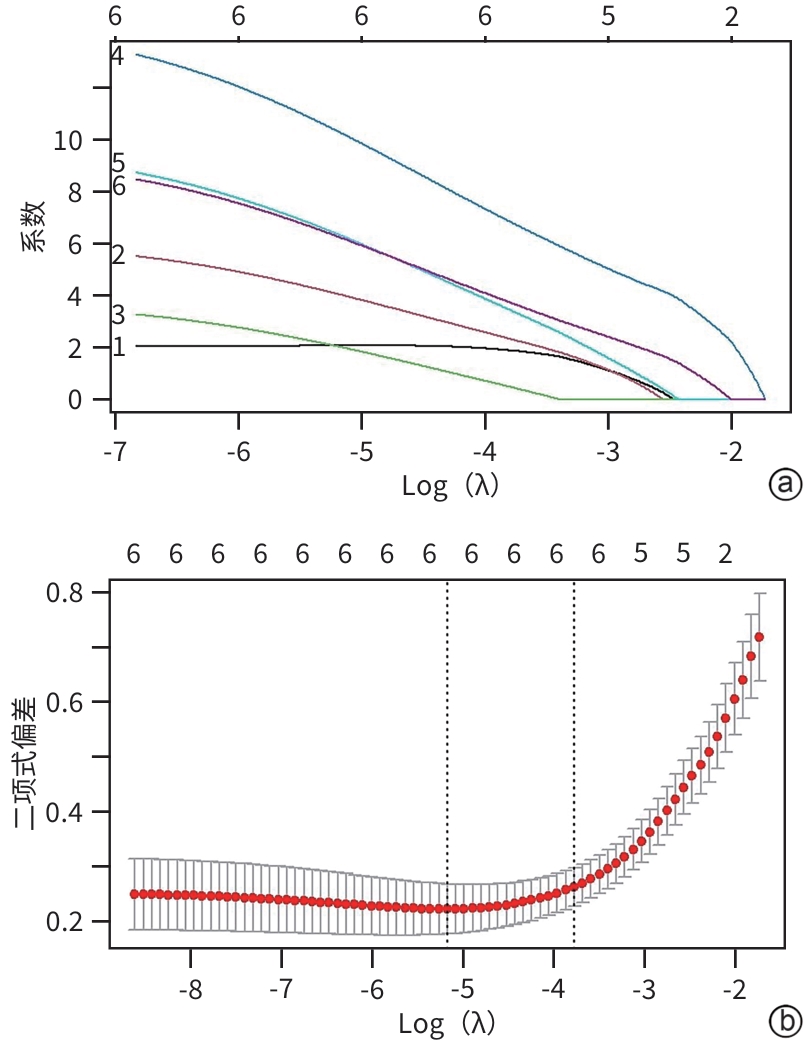
目的 本研究旨在通过分析急性胰腺炎(AP)患者的炎症因子、肺超声评分及CT评分系统,识别AP预后不良的独立危险因素,并构建列线图预测模型,为临床早期干预提供依据。 方法 选取2021年1月—2023年10月苏州大学附属常熟医院收治的409例AP患者为研究对象,使用简单随机抽样法以7∶3分为建模组(n=288)和验证组(n=121)。各组依据转归情况分为预后不良组与预后良好组。于入院72 h内检测患者C反应蛋白(CRP)、降钙素原(PCT)、IL-6、IL-10、TNF-α水平,并在入院48~72 h评估肺超声(LUS)评分、改良CT严重指数(MCTSI)评分和胰腺外炎症CT(EPIC)评分。符合正态分布的计量资料组间比较采用成组t检验;非正态分布的计量资料组间比较采用Mann-Whitney U秩和检验。计数资料组间比较采用χ2检验。使用LASSO回归筛选变量并纳入多因素Logistic回归模型,分析AP预后不良的独立危险因素,构建列线图预测模型,采用受试者操作特征曲线(ROC曲线)和校准曲线评估列线图模型的区分度和拟合优度,决策曲线分析评价预测模型的临床适用性。 结果 288例建模组AP患者中,预后不良组33例(11.46%),预后良好组255例(88.54%);121例验证组AP患者中,预后不良组13例(10.74%),预后良好组108例(89.26%)。建模组中,与预后良好组相比,预后不良组CRP(Z=3.607)、IL-6(Z=4.189)、TNF-α(t=2.584)水平,以及LUS评分(t=8.075)、MCTSI评分(t=5.929)、EPIC评分(t=8.626)均较高(P值均<0.05);多因素Logistic回归分析显示,CRP(OR=3.592,95%CI:1.272~10.138)、IL-6(OR=4.225,95%CI:1.468~12.156)、TNF-α(OR=3.540,95%CI:1.205~10.401)、LUS评分(OR=7.094,95%CI:2.398~20.986)、MCTSI评分(OR=7.612,95%CI:2.832~20.462)及EPIC评分(OR=11.915,95%CI:4.007~35.432)是AP患者发生预后不良的独立危险因素(P值均<0.05)。依据以上6项,建立列线图预测模型,ROC曲线下面积(AUC)为0.924(95%CI:0.883~0.964);最佳截断值的约登指数为0.670,灵敏度为0.909,特异度为0.761;校准曲线显示建模组及验证组的预测结果与观察结果之间均具有良好的一致性;决策曲线分析提示预测模型具有临床有效性。 结论 基于CRP、IL-6、TNF-α、LUS评分、MCTSI评分及EPIC评分构建的AP患者预后不良的风险预测列线图模型有较好的预测效能,可为AP患者临床早期强化治疗方案提供重要策略指导。 Abstract:Objective To investigate the independent risk factors for poor prognosis in patients with acute pancreatitis (AP) by analyzing inflammatory factors, lung ultrasound (LUS) scores, and CT scores, to establish a nomogram prediction model, and to provide a basis for early clinical intervention. Methods A total of 409 patients with AP who were admitted to Changshu Hospital Affiliated to Soochow University from January 2021 to October 2023 were enrolled as subjects, and they were divided into modeling group with 288 patients and validation group with 121 patients using the simple random sampling method at a ratio of 7∶3. According to the prognosis, each group was further divided into poor prognosis group and good prognosis group. The levels of C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) were measured for both groups within 72 hours after admission, and LUS scores, modified CT severity index (MCTSI), and extrapancreatic inflammation on computed tomography (EPIC) scores were assessed within 48 — 72 hours after admission. The independent-samples t test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U rank sum test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A LASSO regression analysis was used to screen for the variables that were included in the multivariate logistic regression model to identify the independent risk factors for the poor prognosis of AP, and then a nomogram prediction model was established. The receiver operating characteristic (ROC) curve and the calibration curve were used to assess the discriminatory ability and goodness of fit of the nomogram model, and a decision curve analysis was used to assess the clinical applicability of the model. Results Among the 288 patients with AP in the modeling group, there were 33 (11.46%) in the poor prognosis group and 255 (88.54%) in the good prognosis group; among the 121 patients with AP in the validation group, there were 13 (10.74%) in the poor prognosis group and 108 (89.26%) in the good prognosis group. Compared with the good prognosis group, the poor prognosis group had significantly higher levels of CRP (Z=3.607, P<0.05), IL-6 (Z=4.189, P<0.05), and TNF-α (t=2.584, P<0.05), and significantly higher scores of LUS (t=8.075, P<0.05), MCTSI (t=5.929, P<0.05), and EPIC (t=8.626, P<0.05). The multivariate logistic regression analysis showed that CRP (odds ratio [OR]=3.592, 95% confidence interval [CI]: 1.272 — 10.138, P<0.05), IL-6 (OR=4.225, 95%CI: 1.468 — 12.156, P<0.05), TNF-α (OR=3.540, 95%CI: 1.205 — 10.401, P<0.05), LUS (OR=7.094, 95%CI: 2.398 — 20.986, P<0.05), MCTSI (OR=7.612, 95%CI: 2.832 — 20.462, P<0.05), and EPIC (OR=11.915, 95%CI: 4.007 — 35.432, P<0.05) were independent risk factor for poor prognosis in patients with AP. A nomogram prediction model was established based on the above 6 indicators, which had an area under the ROC curve of 0.924 (95%CI: 0.883 — 0.964), and the Youden index for the optimal cut-off value was 0.670, with a sensitivity of 0.909 and a specificity of 0.761. The calibration curve showed good consistency between the predicted and observed results in both the modeling group and the validation group. The decision curve analysis showed that the predictive model had certain clinical effectiveness. Conclusion The nomogram model for predicting the risk of poor prognosis in AP patients based on CRP, IL-6, TNF-α, LUS score, MCTSI score, and EPIC score has relatively good predictive performance and can provide important strategic guidance for developing early intensified treatment regimens for AP patients in clinical practice. -
Key words:
- Pancreatitis /
- C-Reactive Protein /
- Interleukins /
- Lung Ultrasound /
- Nomograms
-
表 1 建模组与验证组AP患者基本资料比较
Table 1. Comparison of baseline characteristics between the modeling and validation group of AP patients
指标 建模组(n=288) 验证组(n=121) 统计值 P值 性别[例(%)] χ2=1.273 0.259 男 186(64.58) 71(58.68) 女 102(35.42) 50(41.32) 年龄(岁) 53±17 55±18 t=0.745 0.457 病因[例(%)] χ2=1.077 0.783 胆管疾病 148(51.39) 60(49.59) 高脂血症 114(39.58) 46(38.02) 酒精性 21(7.29) 12(9.91) 其他 5(1.74) 3(2.48) 严重程度[例(%)] χ2=0.193 0.908 轻症 176(61.11) 74(61.16) 中度重症 96(33.33) 39(32.23) 重症 16(5.56) 8(6.61) BMI(kg/m2) 22.16±3.41 21.49±3.57 t=1.767 0.078 CRP(mg/L) 70.15(39.65~94.80) 70.30(39.70~104.70) Z=0.066 0.947 PCT(ng/mL) 0.35(0.19~0.73) 0.32(0.15~0.87) Z=0.960 0.337 IL-6(pg/mL) 77.60(43.85~120.00) 76.10(44.75~112.35) Z=0.021 0.984 IL-10(pg/mL) 31.80(25.20~38.90) 32.90(26.40~38.80) Z=0.637 0.524 TNF-α(pg/mL) 88.43±33.01 84.71±34.03 t=1.029 0.304 LUS评分(分) 8.70±3.10 8.55±2.67 t=0.468 0.640 MCTSI评分(分) 3.85±1.88 3.97±1.92 t=0.584 0.560 EPIC评分(分) 3.17±1.69 3.10±1.68 t=0.369 0.712 表 2 建模组AP患者中预后不良组与预后良好组临床资料比较
Table 2. Comparison of clinical characteristics between the poor and good prognosis groups in the modeling group of AP patients
指标 预后不良组(n=33) 预后良好组(n=255) 统计值 P值 性别[例(%)] χ2=0.071 0.790 男 22(66.67) 164(64.31) 女 11(33.33) 91(35.69) 年龄(岁) 50±18 54±17 t=0.914 0.329 病因[例(%)] χ2=2.240 0.524 胆管疾病 20(60.61) 128(50.20) 高脂血症 10(30.30) 104(40.78) 酒精性 3(9.09) 18(7.06) 其他 0(0) 5(1.96) BMI(kg/m2) 21.97±3.46 22.18±3.41 t=0.804 0.745 CRP(mg/L) 108.30(55.45~187.75) 69.50(38.60~91.40) Z=3.607 <0.001 PCT(ng/mL) 0.48(0.23~1.99) 0.35(0.19~0.50) Z=1.811 0.070 IL-6(pg/mL) 132.00(79.40~203.95) 72.70(41.80~112.30) Z=4.189 <0.001 IL-10(pg/mL) 29.90(23.55~35.70) 32.00(25.30~39.10) Z=1.092 0.275 TNF-α(pg/mL) 108.27±48.66 85.86±29.68 t=2.584 0.014 LUS评分(分) 13.48±3.74 8.09±2.40 t=8.075 <0.001 MCTSI评分(分) 5.58±1.71 3.62±1.79 t=5.929 <0.001 EPIC评分(分) 5.30±1.55 2.89±1.51 t=8.626 <0.001 表 3 预测AP患者发生预后不良的多因素Logistic分析
Table 3. Multivariate logistic analysis of factors for predicting poor prognosis in AP patients
因素 回归系数 标准误 Wald值 P值 OR 95%CI CRP(≤70.15 mg/L=0;>70.15 mg/L=1) 1.279 0.529 5.832 0.016 3.592 1.272~10.138 IL-6(≤77.60 pg/mL=0;>77.60 pg/mL=1) 1.441 0.539 7.142 0.008 4.225 1.468~12.156 TNF-α(≤83.85 pg/mL=0;>83.85 pg/mL=1) 1.264 0.550 5.286 0.021 3.540 1.205~10.401 LUS评分(≤8分=0;>8分=1) 1.959 0.553 12.537 <0.001 7.094 2.398~20.986 MCTSI评分(≤4分=0;>4分=1) 2.030 0.505 16.185 <0.001 7.612 2.832~20.462 EPIC评分(≤3分=0;>3分=1) 2.478 0.556 19.857 <0.001 11.915 4.007~35.432 -
[1] WU SM, ZHOU Q, CAI Y, et al. Development and validation of a prediction model for the early occurrence of acute kidney injury in patients with acute pancreatitis[J]. Ren Fail, 2023, 45( 1): 2194436. DOI: 10.1080/0886022X.2023.2194436. [2] BOXHOORN L, VOERMANS RP, BOUWENSE SA, et al. Acute pancreatitis[J]. Lancet, 2020, 396( 10252): 726- 734. DOI: 10.1016/s0140-6736(20)31310-6. [3] WANG QQ, CHENG YL, ZHOU CC, et al. Clinical characteristics analysis of acute pancreatitis[J]. Chin J Dig Surg, 2023, 22( S1): 38- 43. DOI: 10.3760/cma.j.cn115610-20230908-00073.王琦琦, 程亚丽, 周灿灿, 等. 急性胰腺炎患者的临床特征分析[J]. 中华消化外科杂志, 2023, 22( S1): 38- 43. DOI: 10.3760/cma.j.cn115610-20230908-00073. [4] ZHOU HJ, MEI X, HE XH, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study[J]. Medicine(Baltimore), 2019, 98( 16): e15275. DOI: 10.1097/MD.0000000000015275. [5] WU ZQ, LIU N, WAN YD, et al. Analysis of risk factors affecting renal function recovery in patients with severe acute pancreatitis[J]. Chin J Emerg Med, 2020, 29( 9): 1173- 1177. DOI: 10.3760/cma.j.issn.1671-0282.2020.09.007.吴自谦, 刘楠, 万有栋, 等. 重症急性胰腺炎影响肾功能恢复危险因素分析[J]. 中华急诊医学杂志, 2020, 29( 9): 1173- 1177. DOI: 10.3760/cma.j.issn.1671-0282.2020.09.007. [6] YIN JL, ZHAO MM, WANG Y, et al. Analysis of clinical characteristics and influencing factors of disease prognosis in severe acute pancreatitis at different stages[J]. J Clin Exp Med, 2024, 23( 7): 698- 702. DOI: 10.3969/j.issn.1671-4695.2024.07.007.殷将领, 赵茗茗, 王尧, 等. 不同时期重症急性胰腺炎临床特点及疾病转归的影响因素分析[J]. 临床和实验医学杂志, 2024, 23( 7): 698- 702. DOI: 10.3969/j.issn.1671-4695.2024.07.007. [7] WU YS, GUO F. Management strategies for bleeding complications of severe acute pancreatitis[J]. Chin J Dig Surg, 2023, 22( 11): 1295- 1299. DOI: 10.3760/cma.j.cn115610-20231011-00141.吴银山, 郭丰. 重症急性胰腺炎出血并发症的处理策略[J]. 中华消化外科杂志, 2023, 22( 11): 1295- 1299. DOI: 10.3760/cma.j.cn115610-20231011-00141. [8] SILVA-VAZ P, ABRANTES AM, CASTELO-BRANCO M, et al. Multifactorial scores and biomarkers of prognosis of acute pancreatitis: Applications to research and practice[J]. Int J Mol Sci, 2020, 21( 1): 338. DOI: 10.3390/ijms21010338. [9] van DEN BERG FF, de BRUIJN AC, van SANTVOORT HC, et al. Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis[J]. Pancreatology, 2020, 20( 7): 1302- 1311. DOI: 10.1016/j.pan.2020.09.007. [10] HE J, YU S, ZHANG J. Value of serum interleukin-6 and tumor necrosis factor-α in early diagnosis of severe acute pancreatitis[J]. J Clin Hepatol, 2023, 39( 7): 1657- 1664. DOI: 10.3969/j.issn.1001-5256.2023.07.020.何健, 俞隼, 张静. 血清IL-6和TNF-α对重症急性胰腺炎的早期诊断价值分析[J]. 临床肝胆病杂志, 2023, 39( 7): 1657- 1664. DOI: 10.3969/j.issn.1001-5256.2023.07.020. [11] SKOURAS C, DAVIS ZA, SHARKEY J, et al. Lung ultrasonography as a direct measure of evolving respiratory dysfunction and disease severity in patients with acute pancreatitis[J]. HPB(Oxford), 2015. DOI: 10.1111/hpb.12515. [12] TAYDAS O, UNAL E, KARAOSMANOGLU AD, et al. Accuracy of early CT findings for predicting disease course in patients with acute pancreatitis[J]. Jpn J Radiol, 2018, 36( 2): 151- 158. DOI: 10.1007/s11604-017-0709-9. [13] XU WH, LI XH, YU NJ, et al. Comparison of the imaging and clinical characteristics between initial and recurrent alcoholic acute pancreatitis: A retrospective cross-sectional study[J]. Am J Drug Alcohol Abuse, 2023, 49( 4): 431- 439. DOI: 10.1080/00952990.2023.2211221. [14] Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of acute pancreatitis in China(2021)[J]. Chin J Dig Surg, 2021, 20( 7): 730- 739. DOI: 10.3760/cma.j.cn115610-20210622-00297.中华医学会外科学分会胰腺外科学组. 中国急性胰腺炎诊治指南(2021)[J]. 中华消化外科杂志, 2021, 20( 7): 730- 739. DOI: 10.3760/cma.j.cn115610-20210622-00297. [15] ZHANG J, ZHAO W, REN QL, et al. Predictive value of enhanced CT necrotic volume and attenuation value for poor prognosis of acute necrotizing pancreatitis[J]. Mod Dig Interv, 2021, 26( 12): 1584- 1588. DOI: 10.3969/j.issn.1672-2159.2021.12.024.张景, 赵伟, 任千里, 等. 增强CT坏死体积和衰减值对急性坏死性胰腺炎不良预后的预测价值[J]. 现代消化及介入诊疗, 2021, 26( 12): 1584- 1588. DOI: 10.3969/j.issn.1672-2159.2021.12.024. [16] MONGODI S, de LUCA D, COLOMBO A, et al. Quantitative lung ultrasound: Technical aspects and clinical applications[J]. Anesthesiology, 2021, 134( 6): 949- 965. DOI: 10.1097/ALN.0000000000003757. [17] MORTELE KJ, WIESNER W, INTRIERE L, et al. A modified CT severity index for evaluating acute pancreatitis: Improved correlation with patient outcome[J]. AJR Am J Roentgenol, 2004, 183( 5): 1261- 1265. DOI: 10.2214/ajr.183.5.1831261. [18] de WAELE JJ, DELRUE L, HOSTE EA, et al. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: Evaluation of a new scoring system[J]. Pancreas, 2007, 34( 2): 185- 190. DOI: 10.1097/mpa.0b013e31802d4136. [19] NAWACKI Ł, GŁUSZEK S. Hospital mortality rate and predictors in acute pancreatitis in Poland: A single-center experience[J]. Asian J Surg, 2024, 47( 1): 208- 215. DOI: 10.1016/j.asjsur.2023.07.063. [20] HIDALGO NJ, PANDO E, MATA R, et al. Impact of comorbidities on hospital mortality in patients with acute pancreatitis: A population-based study of 110, 021 patients[J]. BMC Gastroenterol, 2023, 23( 1): 81. DOI: 10.1186/s12876-023-02730-6. [21] LEPPÄNIEMI A, TOLONEN M, TARASCONI A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis[J]. World J Emerg Surg, 2019, 14: 27. DOI: 10.1186/s13017-019-0247-0. [22] AN WH, HE XC, YANG J, et al. Value of early admission scoring systems in predicting the severity and prognosis of acute pancreatitis[J]. J Clin Hepatol, 2020, 36( 6): 1342- 1346. DOI: 10.3969/j.issn.1001-5256.2020.06.030.安文慧, 何旭昶, 杨婧, 等. 入院早期评分系统对急性胰腺炎严重程度及预后的预测价值[J]. 临床肝胆病杂志, 2020, 36( 6): 1342- 1346. DOI: 10.3969/j.issn.1001-5256.2020.06.030. [23] PALIWAL A, NAWAL CL, MEENA PD, et al. A study of procalcitonin as an early predictor of severity in acute pancreatitis[J]. J Assoc Physicians India, 2022, 70( 4): 11- 12. [24] MATHAI MJ, REDDY M VS, SHETTY V. Analysis of the accuracy of the modified CT severity index in predicting clinical outcomes in acute pancreatitis: A cross-sectional study[J]. Cureus, 2024, 16( 3): e56123. DOI: 10.7759/cureus.56123. [25] HU XY, YANG ZY, ZHAO CJ, et al. Research progress in acute pancreatitis scoring systems in predicting the severity of disease[J/OL]. Chin J Hepat Surg(Electronic Edition), 2024, 13( 2): 239- 243. DOI: 10.3877/cma.j.issn.2095-3232.2024.02.021.胡欣芫, 杨智義, 赵成俊, 等. 急性胰腺炎评分系统预测病情严重程度的研究进展[J/OL]. 中华肝脏外科手术学电子杂志, 2024, 13( 2): 239- 243. DOI: 10.3877/cma.j.issn.2095-3232.2024.02.021. [26] STERNBY H, HARTMAN H, JOHANSEN D, et al. IL-6 and CRP are superior in early differentiation between mild and non-mild acute pancreatitis[J]. Pancreatology, 2017, 17( 4): 550- 554. DOI: 10.1016/j.pan.2017.05.392. [27] SUN JX, YAN X. Improved CT severity index combined with IL-6 to predict hospitalization death of acute pancreatitis[J]. Mod Interv Diagn Treat Gastroenterol, 2023, 28( 2): 244- 248. DOI: 10.3969/j.issn.1672-2159.2023.02.023.孙佳欣, 阎曦. 改良CT严重指数联合IL-6预测急性胰腺炎住院死亡[J]. 现代消化及介入诊疗, 2023, 28( 2): 244- 248. DOI: 10.3969/j.issn.1672-2159.2023.02.023. [28] LI P, JIAN JN, CHEN RL. Effect of early enteral nutrition on serum inflammatory factors and intestinal mucosal permeability in patients with severe acute pancreatitis[J]. Turk J Gastroenterol, 2021, 32( 10): 907- 912. DOI: 10.5152/tjg.2021.201033. [29] CARDOSO FS, RICARDO LB, OLIVEIRA AM, et al. C-reactive protein prognostic accuracy in acute pancreatitis: Timing of measurement and cutoff points[J]. Eur J Gastroenterol Hepatol, 2013, 25( 7): 784- 789. DOI: 10.1097/MEG.0b013e32835fd3f0. [30] SAAD H, ERAKY M, EL-TAHE A, et al. A thorough study and meta-analysis of the prognostic relevance of the c-reactive-albumin ratio in acute pancreatitis[J]. Georgian Med News, 2023,( 343): 111- 118. [31] SATHYANARAYAN G, GARG PK, PRASAD H, et al. Elevated level of interleukin-6 predicts organ failure and severe disease in patients with acute pancreatitis[J]. J Gastroenterol Hepatol, 2007, 22( 4): 550- 554. DOI: 10.1111/j.1440-1746.2006.04752.x. [32] HUANG ZY, MA X, JIA XT, et al. Prevention of severe acute pancreatitis with cyclooxygenase-2 inhibitors: A randomized controlled clinical trial[J]. Am J Gastroenterol, 2020, 115( 3): 473- 480. DOI: 10.14309/ajg.0000000000000529. [33] HAN BH, YANG W, WANG H, et al. Construction and evaluation of a prognostic model for severe acute pancreatitis based on CT scores and inflammatory factors[J]. Chin Crit Care Med, 2023, 35( 1): 82- 87. DOI: 10.3760/cma.j.cn121430-20220411-00351.韩宝华, 杨文, 王慧, 等. 基于CT评分和炎症因子的重症急性胰腺炎预后模型构建及评价[J]. 中华危重病急救医学, 2023, 35( 1): 82- 87. DOI: 10.3760/cma.j.cn121430-20220411-00351. [34] SAHU B, ABBEY P, ANAND R, et al. Severity assessment of acute pancreatitis using CT severity index and modified CT severity index: Correlation with clinical outcomes and severity grading as per the Revised Atlanta Classification[J]. Indian J Radiol Imaging, 2017, 27( 2): 152- 160. DOI: 10.4103/ijri.IJRI_300_16. [35] GEZER NS, BENGI G, BARAN A, et al. Comparison of radiological scoring systems, clinical scores, neutrophil-lymphocyte ratio and serum C-reactive protein level for severity and mortality in acute pancreatitis[J]. Rev Assoc Med Bras(1992), 2020, 66( 6): 762- 770. DOI: 10.1590/1806-9282.66.6.762. [36] ZHAO Z, JIANG L, XI XM, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome[J]. BMC Pulm Med, 2015, 15: 98. DOI: 10.1186/s12890-015-0091-2. [37] YANG Q, LUO YL, LAN BW, et al. Fighting fire with fire: Exosomes and acute pancreatitis-associated acute lung injury[J]. Bioengineering(Basel), 2022, 9( 11): 615. DOI: 10.3390/bioengineering9110615. [38] WANG XY, WANG ZP, WU J, et al. Effect of early thoracic paracentesis drainage on acute lung injury in severe acute pancreatitis[J]. J Clin Hepatol, 2023, 39( 7): 1633- 1642. DOI: 10.3969/j.issn.1001-5256.2023.07.018.王旭阳, 王张鹏, 吴俊, 等. 早期胸腔穿刺引流对重症急性胰腺炎急性肺损伤的影响[J]. 临床肝胆病杂志, 2023, 39( 7): 1633- 1642. DOI: 10.3969/j.issn.1001-5256.2023.07.018. [39] RANA SS, KATAGERI B, SHAH J, et al. Role of transthoracic lung ultrasonography in acute pancreatitis[J]. Pancreas, 2020, 49( 5): e47- e48. DOI: 10.1097/MPA.0000000000001556. -



 PDF下载 ( 2126 KB)
PDF下载 ( 2126 KB)

 下载:
下载: