第三腰椎骨骼肌质量指数对慢加急性肝衰竭患者预后的预测价值
DOI: 10.12449/JCH250415
Value of third lumbar skeletal muscle mass index in predicting the prognosis of patients with acute-on-chronic liver failure
-
摘要:
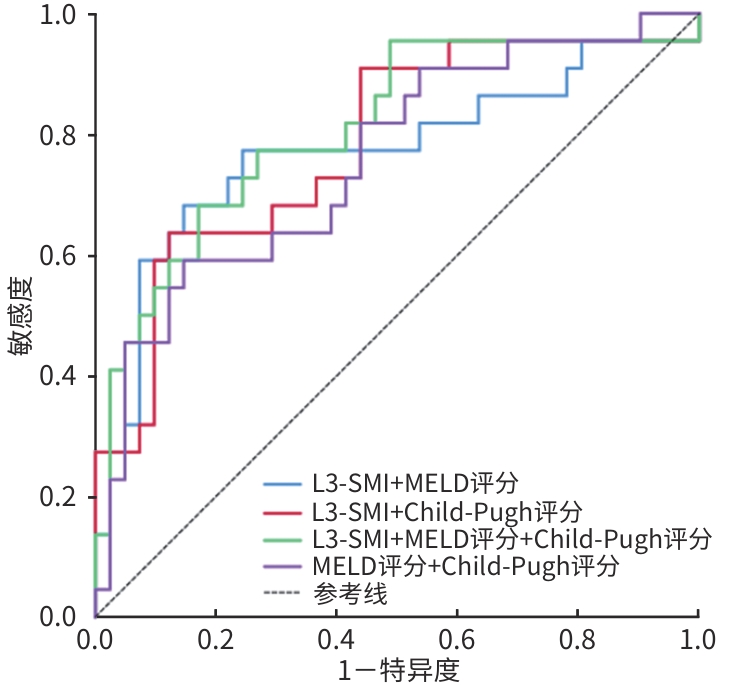
目的 探讨第三腰椎骨骼肌质量指数(L3-SMI)对慢加急性肝衰竭(ACLF)患者长期预后的预测价值,为ACLF患者的预后评分提供实用的工具。 方法 回顾性纳入2017年12月—2021年12月在山西白求恩医院接受腹部CT检查并确诊为ACLF的126例患者,收集患者临床、生化指标以及计算终末期肝病模型(MELD)评分等指标,计算L3-SMI。符合正态分布的计量资料两组间比较采用成组t检验;不符合正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。应用受试者操作特征曲线(ROC曲线)评估L3-SMI和其他变量如MELD评分和Child-Pugh评分的诊断价值,ROC曲线下面积(AUC)的比较使用Delong检验。 结果 126例ACLF患者中有44例(35%)在2年内死亡,82例(65%)存活。死亡组患者年龄、腹水发生率、国际标准化比值、MELD评分、Child-Pugh评分均显著高于存活组(P值均<0.05),L3-SMI显著低于存活组[38.40(35.95~46.29) cm²/m² vs 44.19(40.20~48.58) cm²/m²,Z=-2.855, P=0.004]。L3-SMI预测ACLF患者2年病死率的AUC为0.720,敏感度为63.6%,特异度为80.5%;L3-SMI+MELD评分+Child-Pugh评分联合预测的AUC优于MELD评分+Child-Pugh评分二者联合预测的AUC(0.809 vs 0.757,Z=2.015, P<0.05)。 结论 L3-SMI对ACLF患者的预后有一定的预测作用,且L3-SMI+MELD评分+Child-Pugh评分联合预测ACLF患者预后的价值更高,将L3-SMI或肌肉减少症包括在ACLF患者常规预后的评分中,可能会提高预测疾病进展的能力。 -
关键词:
- 慢加急性肝功能衰竭 /
- 第三腰椎骨骼肌质量指数 /
- 肌减少症
Abstract:Objective To investigate the value of third lumbar skeletal muscle mass index (L3-SMI) in predicting the long-term prognosis of patients with acute-on-chronic liver failure (ACLF), and to provide a useful tool for prognostic scoring of ACLF patients. Methods A retrospective analysis was performed for the data of 126 patients who underwent abdominal computed tomography (CT) scanning and were diagnosed with ACLF in Shanxi Bethune Hospital from December 2017 to December 2021, including clinical indicators, biochemical parameters, and model for end-stage liver disease (MELD) score, and L3-SMI was calculated. The independent-samples t test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. The receiver operating characteristic (ROC) curve was used to assess the diagnostic value of L3-SMI and other variables (MELD score and Child-Pugh score), and the DeLong test was used for comparison of the area under the ROC curve (AUC). Results Among the 126 patients enrolled, 44 (35%) died within 2 years and 82 (65%) survived. Compared with the survival group, the death group had significantly higher age, incidence rate of ascites, international normalized ratio, MELD score, and Child-Pugh score (all P<0.05) and a significantly lower value of L3-SMI [38.40 (35.95 — 46.29) cm²/m² vs 44.19 (40.20 — 48.58) cm²/m², Z=-2.855, P=0.004]. L3-SMI had an AUC of 0.720 in predicting 2-year mortality in ACLF patients, with a sensitivity of 63.6% and a specificity of 80.5%, and a combination of L3-SMI, MELD score, and Child-Pugh score had a significantly better AUC than a combination of MELD score and Child-Pugh score in predicting 2-year mortality (0.809 vs 0.757, Z=2.015, P<0.05). Conclusion L3-SMI has a high predictive value for the prognosis of ACLF patients, and the combination of L3-SMI、MELD score and Child-Pugh score has a higher predictive value for ACLF patients, and the inclusion of L3-SMI or sarcopenia in the conventional prognostic scores of ACLF patients may increase the ability to predict disease progression. -
表 1 ACLF存活与非存活患者的基线特征
Table 1. Baseline characteristics of patients who survived and did not survive acute-on-chronic liver failure
项目 死亡组(n=44) 存活组(n=82) 统计值 P值 性别[例(%)] χ2=1.244 0.571 男 30(68.2) 50(61.0) 女 14(31.8) 32(39.0) 年龄(岁) 55.05±10.20 47.59±10.81 t=3.299 0.010 体质量(kg) 62.94±7.45 65.37±11.68 t=-0.514 0.443 BMI(kg/m2) 22.74±3.53 23.22±3.74 t=-0.230 0.658 病因[例(%)] HBV 16(36.4) 40(48.8) χ2=0.894 0.344 酒精相关性 20(45.5) 16(19.5) χ2=4.722 0.030 自身免疫性 4(9.1) 8(9.8) χ2=0.007 0.932 其他 4(9.1) 18(22.0) χ2=1.643 0.302 RBC(×1012/L) 3.17±0.85 3.60±0.88 t=-1.766 0.062 WBC(×109/L) 7.05(4.40~10.90) 6.30(4.75~9.00) Z=-1.348 0.531 Hb(g/L) 108.14±20.87 118.24±24.75 t=-1.500 0.109 PLT(×109/L) 84.50(50.50~113.25) 113.00(78.00~150.50) Z=-1.752 0.068 TBil(μmol/L) 323.00(251.70~487.93) 285.20(193.25~408.10) Z=-1.384 0.235 Alb(g/L) 27.62±4.67 29.54±5.83 t=-1.839 0.188 Cr(μmol/L) 106.95(65.65~179.00) 77.25(68.75~121.35) Z=-2.251 0.117 INR 2.23(1.89~2.87) 1.82(1.60~2.42) Z=-2.733 0.008 Child-Pugh评分(分) 12.00(10.00~13.00) 11.00(9.00~12.00) Z=-2.799 0.007 MELD评分(分) 27.54±8.11 22.82±7.09 t=3.192 0.020 腹水[例(%)] 40(90.9) 52(63.4) χ2=5.493 0.019 肝性脑病[例(%)] 16(36.4) 16(19.5) χ2=2.146 0.219 肝肾综合征[例(%)] 6(13.6) 4(4.9) χ2=1.503 0.333 L3-SMI(cm²/m²) 38.40(35.95~46.29) 44.19(40.20~48.58) Z=-2.855 0.004 注:INR,国际标准化比值。
表 2 各项指标及联合指标的诊断效能
Table 2. Diagnostic efficacy of individual indicators and combined indicators
指标 AUC 95%CI 截断值 敏感度(%) 特异度(%) P值 Z值1) P值1) L3-SMI 0.720 0.577~0.862 39.52 63.6 80.5 0.004 1.145 0.145 Child-Pugh评分 0.712 0.574~0.846 11.50 54.5 78.0 0.006 2.038 0.040 MELD评分 0.728 0.592~0.864 25.34 63.6 75.6 0.003 1.749 0.080 L3-SMI+MELD评分 0.771 0.633~0.906 0.63 68.2 85.4 <0.001 1.228 0.219 L3-SMI+Child-Pugh评分 0.783 0.659~0.906 0.56 63.6 87.8 <0.001 0.898 0.369 L3-SMI+MELD评分+Child-Pugh评分 0.809 0.691~0.927 0.60 68.2 82.9 <0.001 MELD+Child-Pugh评分 0.757 0.630~0.884 0.56 59.1 85.4 <0.001 2.015 0.046 注:1)与L3-SMI+MELD评分+Child-Pugh评分比较。
-
[1] HERNAEZ R, KRAMER JR, LIU Y, et al. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA[J]. J Hepatol, 2019, 70( 4): 639- 647. DOI: 10.1016/j.jhep.2018.12.018. [2] ZHANG B, DILIHUMAER ZYE, ZHANG SY, et al. Progress on pathogenesis and medical treatment of hepatitis B virus-related chronic and acute liver failure[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005.张斌, 迪丽胡玛尔·扎依尔, 张诗雨, 等. 乙型肝炎相关慢加急性肝衰竭发病机制及治疗进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005. [3] ABDALLAH MA, KUO YF, ASRANI S, et al. Validating a novel score based on interaction between ACLF grade and MELD score to predict waitlist mortality[J]. J Hepatol, 2021, 74( 6): 1355- 1361. DOI: 10.1016/j.jhep.2020.12.003. [4] ROLLAND Y, CZERWINSKI S, ABELLAN VAN KAN G, et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives[J]. J Nutr Health Aging, 2008, 12( 7): 433- 450. DOI: 10.1007/BF02982704. [5] KIM G, KANG SH, KIM MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis[J]. PLoS One, 2017, 12( 10): e0186990. DOI: 10.1371/journal.pone.0186990. [6] CHAN HCN, FEI XZ, LEUNG ELY, et al. Post-discharge consequences of protein-energy malnutrition, sarcopenia, and frailty in older adults admitted to rehabilitation: A systematic review[J]. Clin Nutr ESPEN, 2023, 54: 382- 397. DOI: 10.1016/j.clnesp.2023.01.023. [7] TANTAI XX, LIU Y, YEO YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis[J]. J Hepatol, 2022, 76( 3): 588- 599. DOI: 10.1016/j.jhep.2021.11.006. [8] van VUGT JLA, ALFERINK LJM, BUETTNER S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort[J]. J Hepatol, 2018, 68( 4): 707- 714. DOI: 10.1016/j.jhep.2017.11.030. [9] YAO J, ZHOU XS, YUAN LL, et al. Prognostic value of the third lumbar skeletal muscle mass index in patients with liver cirrhosis and ascites[J]. Clin Nutr, 2020, 39( 6): 1908- 1913. DOI: 10.1016/j.clnu.2019.08.006. [10] KONG M, GENG N, ZHOU Y, et al. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: A multicentre study[J]. Clin Nutr, 2022, 41( 2): 396- 404. DOI: 10.1016/j.clnu.2021.12.003. [11] LIDORIKI I, SCHIZAS D, MPAILI E, et al. Associations between skeletal muscle mass index, nutritional and functional status of patients with oesophago-gastric cancer[J]. Clin Nutr ESPEN, 2019, 34: 61- 67. DOI: 10.1016/j.clnesp.2019.08.012. [12] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [13] CAREY EJ, LAI JC, WANG CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease[J]. Liver Transpl, 2017, 23( 5): 625- 633. DOI: 10.1002/lt.24750. [14] DURAND F, BUYSE S, FRANCOZ C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography[J]. J Hepatol, 2014, 60( 6): 1151- 1157. DOI: 10.1016/j.jhep.2014.02.026. [15] MONTANO-LOZA AJ, DUARTE-ROJO A, MEZA-JUNCO J, et al. Inclusion of sarcopenia within MELD(MELD-sarcopenia) and the prediction of mortality in patients with cirrhosis[J]. Clin Transl Gastroenterol, 2015, 6( 7): e102. DOI: 10.1038/ctg.2015.31. [16] KAMATH PS, KIM WR. The model for end-stage liver disease(MELD)[J]. Hepatology, 2007, 45( 3): 797- 805. DOI: 10.1002/hep.21563. [17] STEWART CA, MALINCHOC M, KIM W RAY, et al. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease[J]. Liver Transpl, 2007, 13( 10): 1366- 1371. DOI: 10.1002/lt.21129. [18] van VUGT JA, LEVOLGER S, de BRUIN RF, et al. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation[J]. Am J Transplant, 2016, 16( 8): 2277- 2292. DOI: 10.1111/ajt.13732. [19] MYERS RP, TANDON P, NEY M, et al. Validation of the five-variable Model for End-stage Liver Disease(5vMELD) for prediction of mortality on the liver transplant waiting list[J]. Liver Int, 2014, 34( 8): 1176- 1183. DOI: 10.1111/liv.12373. [20] FRONTERA WR, OCHALA J. Skeletal muscle: A brief review of structure and function[J]. Calcif Tissue Int, 2015, 96( 3): 183- 195. DOI: 10.1007/s00223-014-9915-y. [21] SAM J, NGUYEN GC. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension[J]. Liver Int, 2009, 29( 9): 1396- 1402. DOI: 10.1111/j.1478-3231.2009.02077.x. [22] WRIGHT G, NOIRET L, OLDE DAMINK SWM, et al. Interorgan ammonia metabolism in liver failure: The basis of current and future therapies[J]. Liver Int, 2011, 31( 2): 163- 175. DOI: 10.1111/j.1478-3231.2010.02302.x. [23] REN PP, XIE Q. Treatment and prevention of hepatic encephalopathy with muscle as the target[J]. Chin Hepatol, 2017, 22( 3): 194- 195. DOI: 10.14000/j.cnki.issn.1008-1704.2017.03.002.任佩佩, 谢青. 以肌肉为靶点的肝性脑病治疗和预防[J]. 肝脏, 2017, 22( 3): 194- 195. DOI: 10.14000/j.cnki.issn.1008-1704.2017.03.002. [24] LI JH, YAO J, YUAN LL. Association between sarcopenia and hepatic encephalopathy and advances in diagnosis and treatment[J]. J Clin Hepatol, 2020, 36( 6): 1412- 1414. DOI: 10.3969/j.issn.1001-5256.2020.06.049.李建宏, 姚佳, 原丽莉. 肌肉减少症与肝性脑病的关系及诊疗进展[J]. 临床肝胆病杂志, 2020, 36( 6): 1412- 1414. DOI: 10.3969/j.issn.1001-5256.2020.06.049. [25] PENG H, ZHANG Q, LUO L, et al. A prognostic model of acute-on-chronic liver failure based on sarcopenia[J]. Hepatol Int, 2022, 16( 4): 964- 972. DOI: 10.1007/s12072-022-10363-2. -



 PDF下载 ( 1075 KB)
PDF下载 ( 1075 KB)


 下载:
下载: