同源盒基因A6(HOXA6)调控肝癌HepG2细胞增殖、侵袭、转移和凋亡的作用机制
DOI: 10.12449/JCH250414
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:刘玉婷、侯天禄负责实验操作;麦静愔负责数据统计分析;成扬负责设计研究方案,指导撰写文章并最后定稿。
Mechanism of action of Homebox A6 in regulating the proliferation, invasion, metastasis, and apoptosis of HepG2 hepatoma cells
-
摘要:
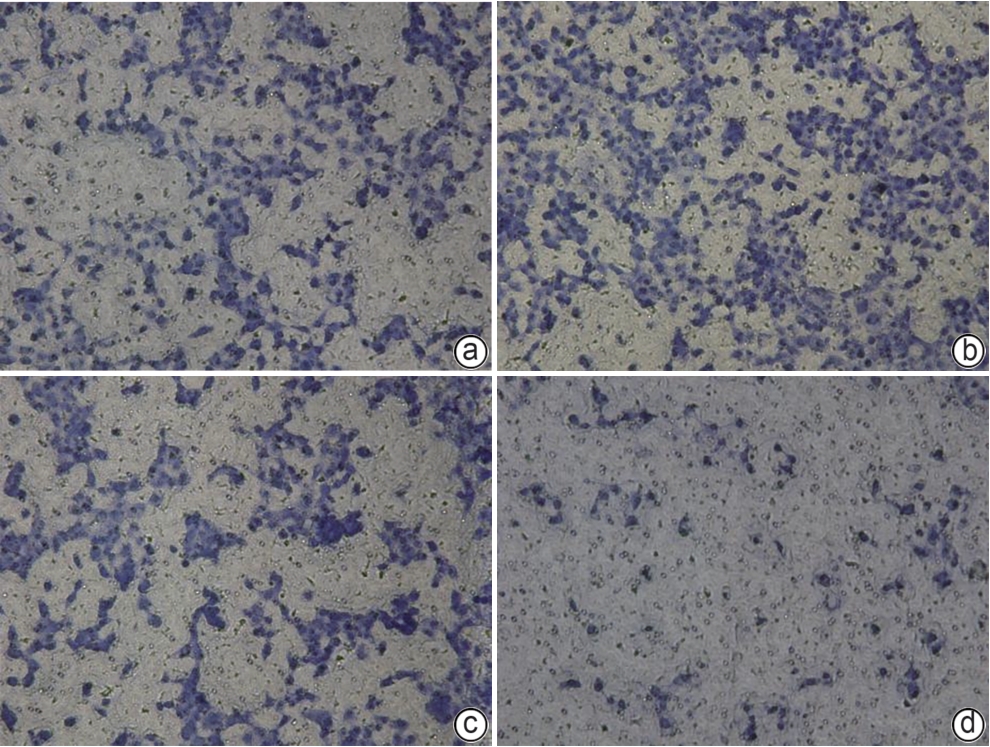
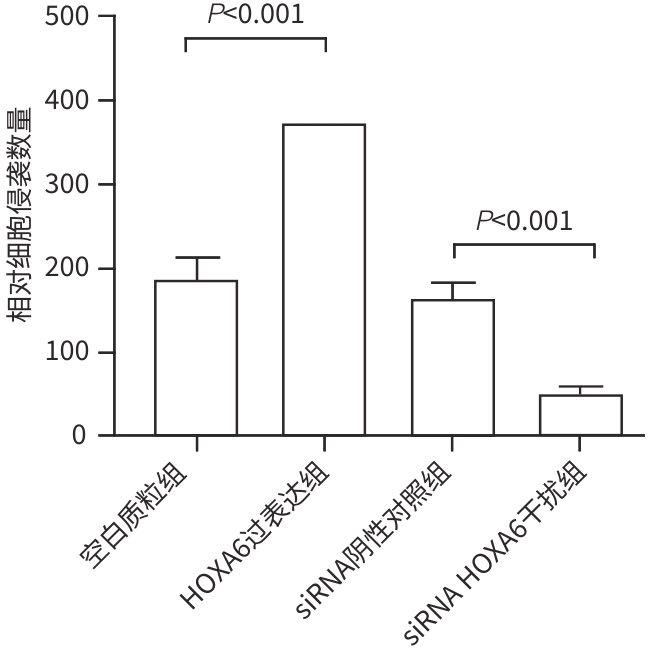
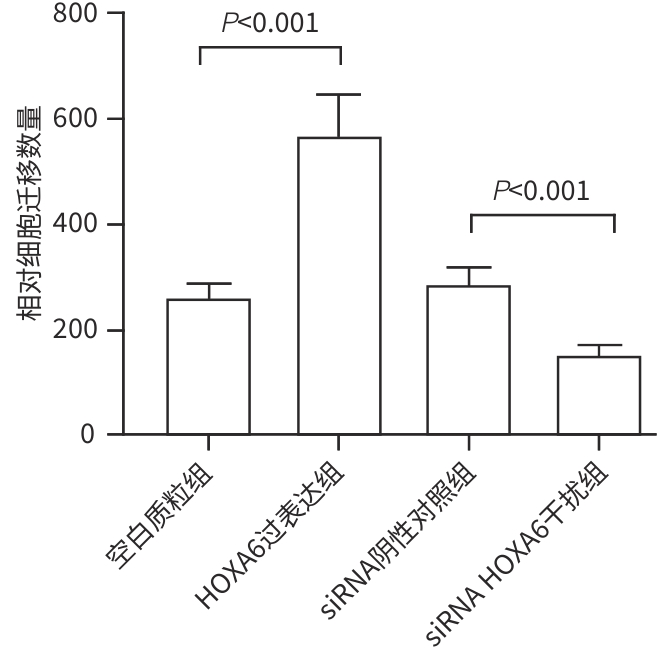
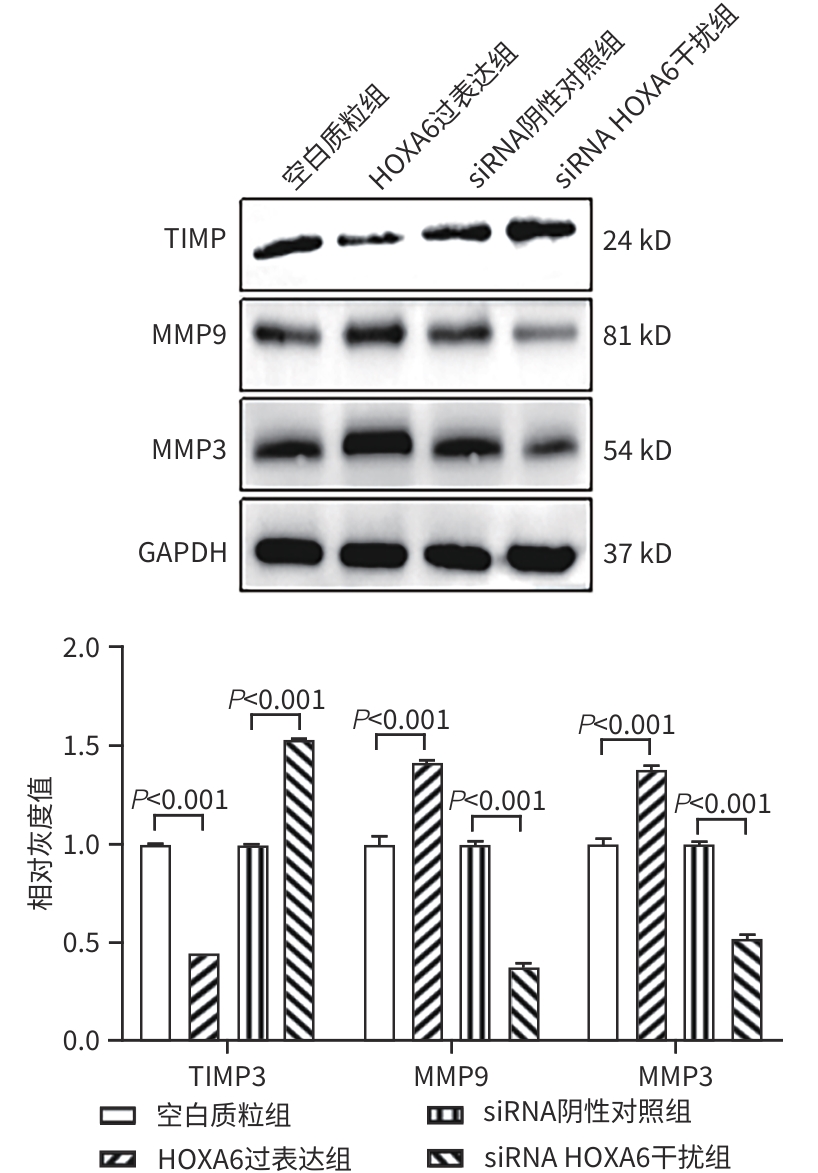
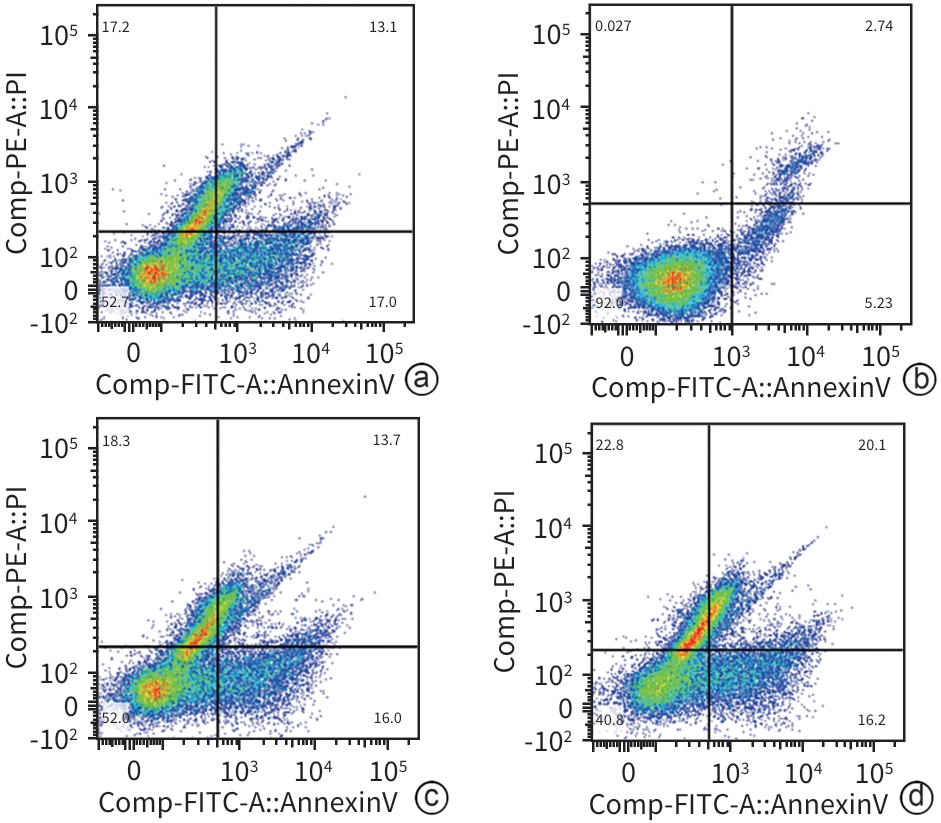
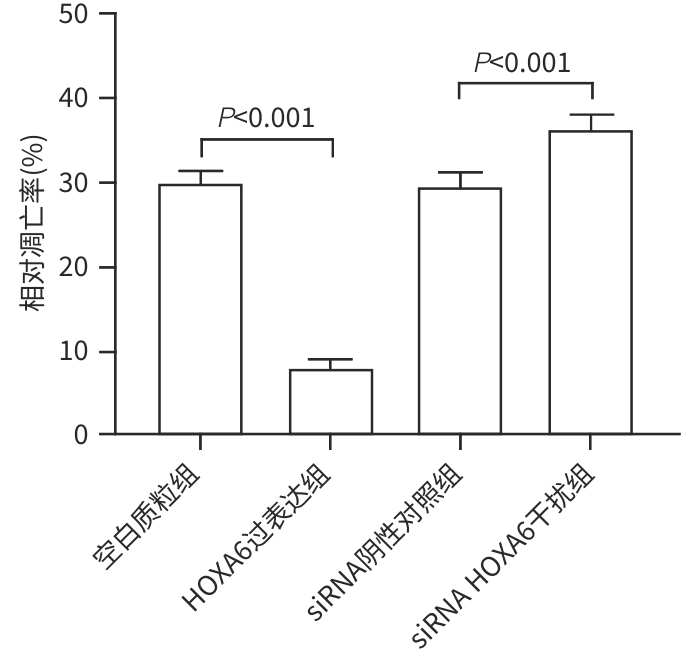
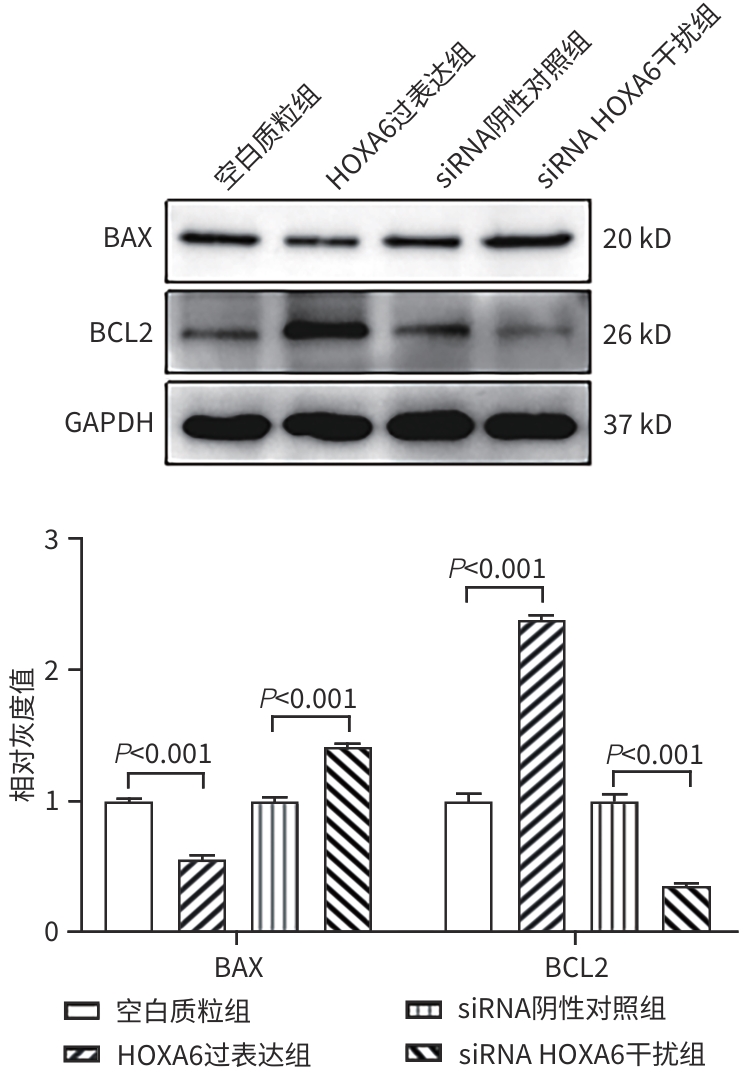
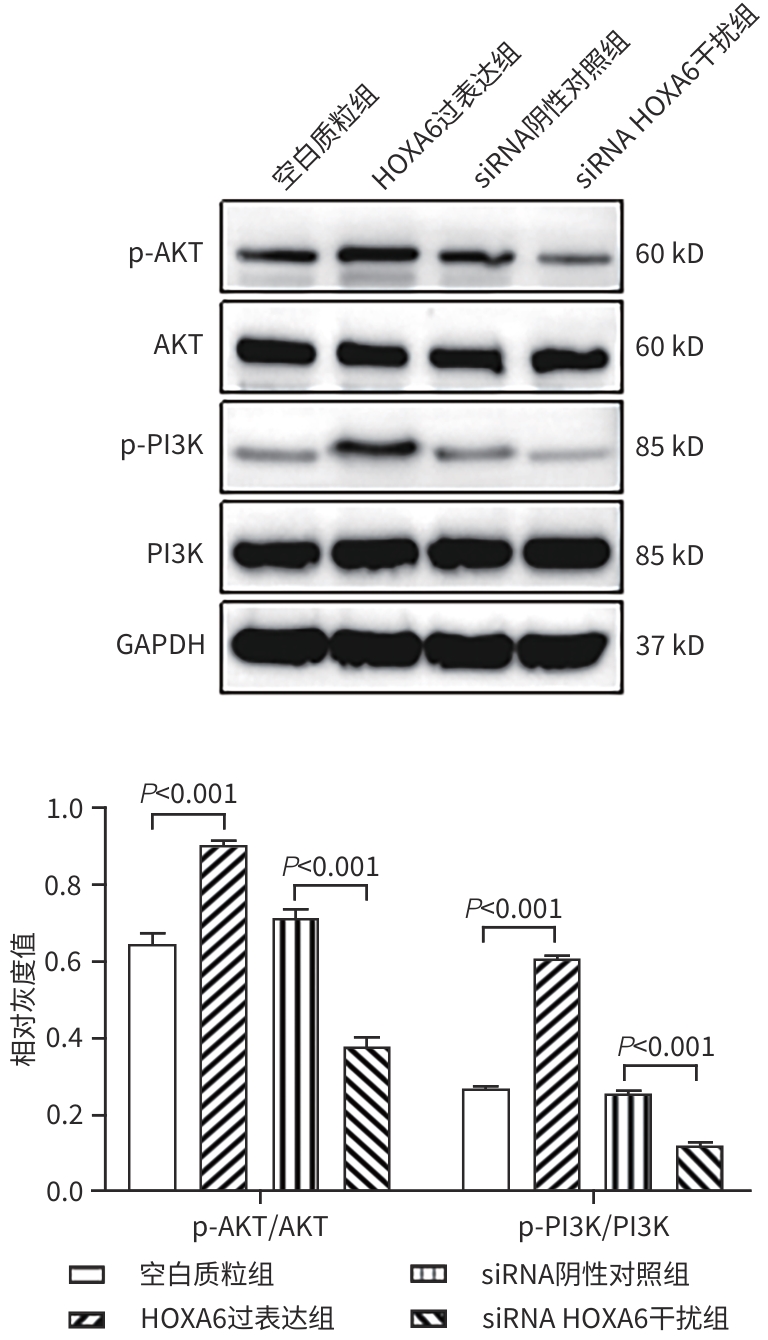
目的 研究同源盒基因A6(HOXA6)对肝癌HepG2细胞增殖、侵袭、转移和凋亡的影响,以及与PI3K/AKT信号通路的关系。 方法 培养肝癌HepG2细胞,构建HOXA6过表达质粒和干扰siRNA并转染细胞,随机分成4组:空白质粒组、HOXA6过表达组、siRNA阴性对照组和siRNA HOXA6干扰组。采用CCK-8法检测细胞增殖,Transwell法检测细胞侵袭,划痕愈合实验检测细胞迁移(相关蛋白TIMP3、MMP9和MMP3),流式细胞仪检测HepG2肝癌细胞凋亡(相关蛋白BAX和BCL2),BCA法测定蛋白质浓度,Western Blot检测蛋白表达水平。计量资料多组间比较采用单因素方差分析,进一步两两比较采用SNK-q检验。 结果 与空白质粒组比,HOXA6过表达显著促进了肝癌HepG2细胞的增殖、侵袭和迁移(P值均<0.001);TIMP3蛋白表达水平显著下降(P<0.001),而MMP9和MMP3表达水平均显著升高(P值均<0.001)。与siRNA阴性对照组比,干扰HOXA6表达显著抑制了肝癌HepG2细胞的增殖、侵袭和迁移(P值均<0.001);TIMP3蛋白表达水平显著升高(P<0.001),MMP9和MMP3表达均显著下降(P值均<0.001)。流式细胞仪检测结果显示,与空白质粒组比,HOXA6过表达抑制了肝癌HepG2细胞的凋亡(P<0.001);凋亡相关蛋白BAX表达下降而BCL2表达升高(P值均<0.001)。与siRNA阴性对照组相比,干扰HOXA6表达则促进了肝癌HepG2细胞的凋亡(P<0.001);BAX表达升高而BCL2表达下降(P值均<0.001)。HOXA6过表达组p-AKT/AKT和p-PI3K/PI3K均显著高于空白质粒组(P值均<0.001),siRNA HOXA6干扰组p-AKT/AKT和p-PI3K/PI3K均显著低于siRNA阴性对照组(P值均<0.001)。 结论 HOXA6通过磷酸化激活PI3K/AKT信号通路促进HepG2肝癌细胞增殖、侵袭和转移,抑制肝癌细胞凋亡。 Abstract:Objective To investigate the effect of Homebox A6 (HOXA6) on the proliferation, invasion, metastasis, and apoptosis of HepG2 hepatoma cells and its association with the PI3K/AKT signaling pathway. Methods HepG2 hepatoma cells were cultured, and HOXA6 overexpression plasmid and siRNA were constructed and transfected into cells. The cells were randomly divided into empty plasmid group, HOXA 6 overexpression group, siRNA negative control group, and siRNA HOXA6 interference group. CCK8 assay was used to measure cell proliferation, Transwell assay was used to observe cell invasion, and wound healing assay was used to observe cell migration (related proteins TIMP3, MMP9, and MMP3). Flow cytometry was used to measure cell apoptosis (related proteins BAX and BCL2), the BCA method was used to measure protein concentration, and Western Blot was used to measure the expression of related proteins. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the SNK-q test was used for further comparison between two groups. Results Compared with the empty plasmid group, HOXA6 overexpression significantly promoted the proliferation, invasion, and migration of HepG2 hepatoma cells (all P<0.001), and there was a significant reduction in the protein expression of TIMP3 (P<0.001), while there were significant increases in the expression levels of MMP9 and MMP3 (both P<0.001). Compared with the siRNA negative control group, HOXA6 interference significantly inhibited the proliferation, invasion, and migration of HepG2 hepatoma cells (all P<0.001), and there was a significant increase in the protein expression of TIMP3 (P<0.001), while there were significant reductions in the expression levels of MMP9 and MMP3 (both P<0.001). Flow cytometry showed that compared with the empty plasmid group, HOXA6 overexpression inhibited the apoptosis of HepG2 hepatoma cells (P<0.001), with a significant reduction in the expression of the apoptosis-related protein BAX and a significant increase in the expression of BCL2 (both P<0.001). Compared with siRNA negative control group, HOXA6 interference promoted the apoptosis of HepG2 hepatoma cells (P<0.001), with a significant increase in the expression of BAX and a significant reduction in the expression of BCL2 (both P<0.001). Compared with the empty plasmid group, the HOXA6 overexpression group had significantly higher ratios of p-AKT/AKT and p-PI3K/PI3K (both P<0.001), and compared with the siRNA negative control group, the siRNA HOXA6 interference group had significantly lower ratios of p-AKT/AKT and p-PI3K/PI3K (both P<0.001). Conclusion HOXA6 can promote the proliferation, invasion, and metastasis of HepG2 hepatoma cells and inhibit their apoptosis by activating the PI3K/AKT signaling pathway through phosphorylation. -
Key words:
- Hep G2 Cells /
- Genes, Homeobox /
- Cell Proliferation /
- Apoptosis
-
-
[1] LIU Y, ZHENG JX, HAO JL, et al. Global burden of primary liver cancer by five etiologies and global prediction by 2035 based on global burden of disease study 2019[J]. Cancer Med, 2022, 11( 5): 1310- 1323. DOI: 10.1002/cam4.4551. [2] YANG XL, XUE JH, CHEN TY, et al. Effect of atractylone on the viability and apoptosis of hepatoma HepG2 cells and related mechanism[J]. J Clin Hepatol, 2021, 37( 11): 2589- 2594. DOI: 10.3969/j.issn.1001-5256.2021.11.020.杨雪丽, 薛建华, 陈天阳, 等. 苍术酮对肝癌HepG2细胞活性、凋亡的影响及其相关机制[J]. 临床肝胆病杂志, 2021, 37( 11): 2589- 2594. DOI: 10.3969/j.issn.1001-5256.2021.11.020. [3] XIE LH, WANG FL, LIU JJ. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 5): 586- 590. DOI: 10.13463/j.cnki.cczyy.2024.05.026.谢林虎, 王凤玲, 刘建军. 原发性肝癌介入联合免疫治疗的研究进展[J]. 长春中医药大学学报, 2024, 40( 5): 586- 590. DOI: 10.13463/j.cnki.cczyy.2024.05.026. [4] DENG N, LI XM, DING XY, et al. Current status and progress of second-line treatment for hepatocellular carcinoma[J/CD]. Chin J Liver Dis(Electronic Version), 2024, 16( 1): 1- 6. DOI: 10.3969/j.issn.1674-7380.2024.01.001.邓娜, 栗晓咪, 丁晓燕, 等. 肝细胞癌二线治疗的现状和进展[J/CD]. 中国肝脏病杂志(电子版), 2024, 16( 1): 1- 6. DOI: 10.3969/j.issn.1674-7380.2024.01.001. [5] XU HC, WANG FL, XIE LH. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024.许华晨, 王凤玲, 谢林虎. 中晚期原发性肝细胞癌的临床治疗现状与展望[J]. 长春中医药大学学报, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024. [6] MAI JY, CHEN S, KE BL, et al. Effects of atractylolone on tumor growth, epithelial mesenchymal transition and expression of apoptosis markers in hepatoma-bearing mice[J]. Inf Tradit Chin Med, 2022, 39( 6): 28- 32. DOI: 10.19656/j.cnki.1002-2406.20220606.麦静愔, 陈澍, 柯碧莲, 等. 苍术酮对肝癌荷瘤小鼠肿瘤生长、上皮-间充质转化和凋亡标志物表达的影响[J]. 中医药信息, 2022, 39( 6): 28- 32. DOI: 10.19656/j.cnki.1002-2406.20220606. [7] LIU HB, LUO SC, SHA XF, et al. Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts through regulation of the HOXA6/ZBTB12 axis[J]. Acta Pharm, 2023, 73( 3): 423- 439. DOI: 10.2478/acph-2023-0033. [8] JIANG XL, WANG CY, GUO JS, et al. Next-generation sequencing identifies HOXA6 as a novel oncogenic gene in low grade glioma[J]. Aging(Albany NY), 2022, 14( 6): 2819- 2854. DOI: 10.18632/aging.203977. [9] JIN ZP, SUN DX, SONG MY, et al. Comprehensive analysis of HOX family members as novel diagnostic and prognostic markers for hepatocellular carcinoma[J]. J Oncol, 2022, 2022: 5758601. DOI: 10.1155/2022/5758601. [10] WAN CY, HOU TL, CHENG Y. Huangqi decoction serum suppressed proliferation and migration of rat liver sinusoidal endothelial cells via angiogenesis inhibition by promoting AKT/mTOR-dependent autophagy[J]. Indian J Pharm Sci, 2024, 86( 3): 1058- 1068. DOI: 10.36468/pharmaceutical-sciences.1364. [11] SKALA MC, RICHING KM, BIRD DK, et al. In vivo multiphoton fluorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia[J]. J Biomed Opt, 2007, 12( 2): 024014. DOI: 10.1117/1.2717503. [12] LIN JJ, ZHU HQ, HONG LJ, et al. Coexpression of HOXA6 and PBX2 promotes metastasis in gastric cancer[J]. Aging(Albany NY), 2021, 13( 5): 6606- 6624. DOI: 10.18632/aging.202426. [13] DENG J, DING HH, LONG JL, et al. Oxaliplatin-induced neuropathic pain involves HOXA6 via a TET1-dependent demethylation of the SOX10 promoter[J]. Int J Cancer, 2020, 147( 9): 2503- 2514. DOI: 10.1002/ijc.33106. [14] DU YX, XU YQ, GUO XF, et al. Methylation-regulated tumor suppressor gene PDE7B promotes HCC invasion and metastasis through the PI3K/AKT signaling pathway[J]. BMC Cancer, 2024, 24( 1): 624. DOI: 10.1186/s12885-024-12364-w. [15] WANG HY, MAI JY, PING J, et al. Effect of serum containing Huangqi decoction on the proliferation, migration, and tubulogenesis of rat liver sinusoidal endothelial cells induced by vascular endothelial growth factor and its mechanism of action[J]. J Clin Hepatol, 2022, 38( 10): 2279- 2285. DOI: 10.3969/j.issn.1001-5256.2022.10.015.王浩艺, 麦静愔, 平键, 等. 黄芪汤含药血清对血管内皮生长因子诱导的大鼠肝窦内皮细胞增殖、迁移和成管能力的影响及其作用机制[J]. 临床肝胆病杂志, 2022, 38( 10): 2279- 2285. DOI: 10.3969/j.issn.1001-5256.2022.10.015. [16] GALASSO L, CERRITO L, MACCAURO V, et al. Inflammatory response in the pathogenesis and treatment of hepatocellular carcinoma: A double-edged weapon[J]. Int J Mol Sci, 2024, 25( 13): 7191. DOI: 10.3390/ijms25137191. [17] YANG JD, HAINAUT P, GORES GJ, et al. A global view of hepatocellular carcinoma: Trends, risk, prevention and management[J]. Nat Rev Gastroenterol Hepatol, 2019, 16( 10): 589- 604. DOI: 10.1038/s41575-019-0186-y. [18] PINTER M, PINATO DJ, RAMADORI P, et al. NASH and hepatocellular carcinoma: Immunology and immunotherapy[J]. Clin Cancer Res, 2023, 29( 3): 513- 520. DOI: 10.1158/1078-0432.CCR-21-1258. [19] HUI YF, LENG JZ, JIN D, et al. BRG1 promotes liver cancer cell proliferation and metastasis by enhancing mitochondrial function and ATP5A1 synthesis through TOMM40[J]. Cancer Biol Ther, 2024, 25( 1): 2375440. DOI: 10.1080/15384047.2024.2375440. [20] ADETUTU A, ADEGBOLA PI, ABORISADE AB. Low dose of nickel and benzo[a] anthracene in rat-diet, induce apoptosis, fibrosis, and initiate carcinogenesis in liver via NF-κβ pathway[J]. Biol Trace Elem Res, 2025, 203( 1): 305- 333. DOI: 10.1007/s12011-024-04177-6. [21] ASHRAF TS, OBAID A, SAEED TM, et al. Formal model of the interplay between TGFβ1 and MMP-9 and their dynamics in hepatocellular carcinoma[J]. Math Biosci Eng, 2019, 16( 5): 3285- 3310. DOI: 10.3934/mbe.2019164. [22] SCHEAU C, BADARAU IA, COSTACHE R, et al. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma[J]. Anal Cell Pathol(Amst), 2019, 2019: 9423907. DOI: 10.1155/2019/9423907. [23] TANAKA H, OKAMOTO K, SATO Y, et al. Synergistic anti-tumor activity of miriplatin and radiation through PUMA-mediated apoptosis in hepatocellular carcinoma[J]. J Gastroenterol, 2020, 55( 11): 1072- 1086. DOI: 10.1007/s00535-020-01705-8. [24] LEE J, KIM J, LEE R, et al. SOD1 inhibition enhances sorafenib efficacy in HBV-related hepatocellular carcinoma by modulating PI3K/Akt/mTOR pathway and ROS-mediated cell death[J]. J Cell Mol Med, 2024, 28( 14): e18533. DOI: 10.1111/jcmm.18533. [25] LUO BY, ZHUANG L, HUANG J, et al. LncRNA ZFAS1 regulates ATIC transcription and promotes the proliferation and migration of hepatocellular carcinoma through the PI3K/AKT signaling pathway[J]. J Cancer Res Clin Oncol, 2024, 150( 7): 351. DOI: 10.1007/s00432-024-05877-1. [26] ZHANG YC, LIANG JJ, CAO ND, et al. ASIC1α up-regulates MMP-2/9 expression to enhance mobility and proliferation of liver cancer cells via the PI3K/AKT/mTOR pathway[J]. BMC Cancer, 2022, 22( 1): 778. DOI: 10.1186/s12885-022-09874-w. [27] WU FX, WU SS, TONG H, et al. HOXA6 inhibits cell proliferation and induces apoptosis by suppressing the PI3K/Akt signaling pathway in clear cell renal cell carcinoma[J]. Int J Oncol, 2019, 54( 6): 2095- 2105. DOI: 10.3892/ijo.2019.4789. -



 PDF下载 ( 8150 KB)
PDF下载 ( 8150 KB)


 下载:
下载: