血清醛酮还原酶家族1成员B10(AKR1B10)对肝细胞癌的诊断价值
DOI: 10.12449/JCH250413
Value of serum Aldo-keto reductase family 1 member B10 (AKR1B10) in diagnosis of hepatocellular carcinoma
-
摘要:
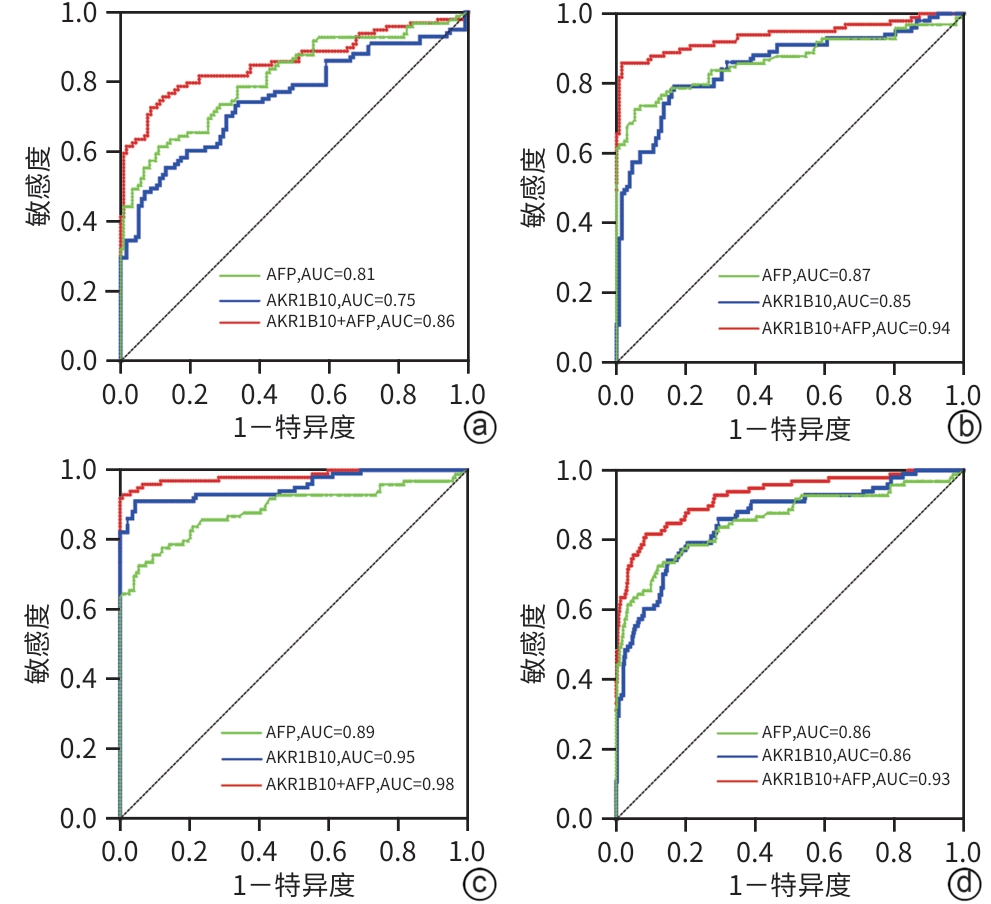
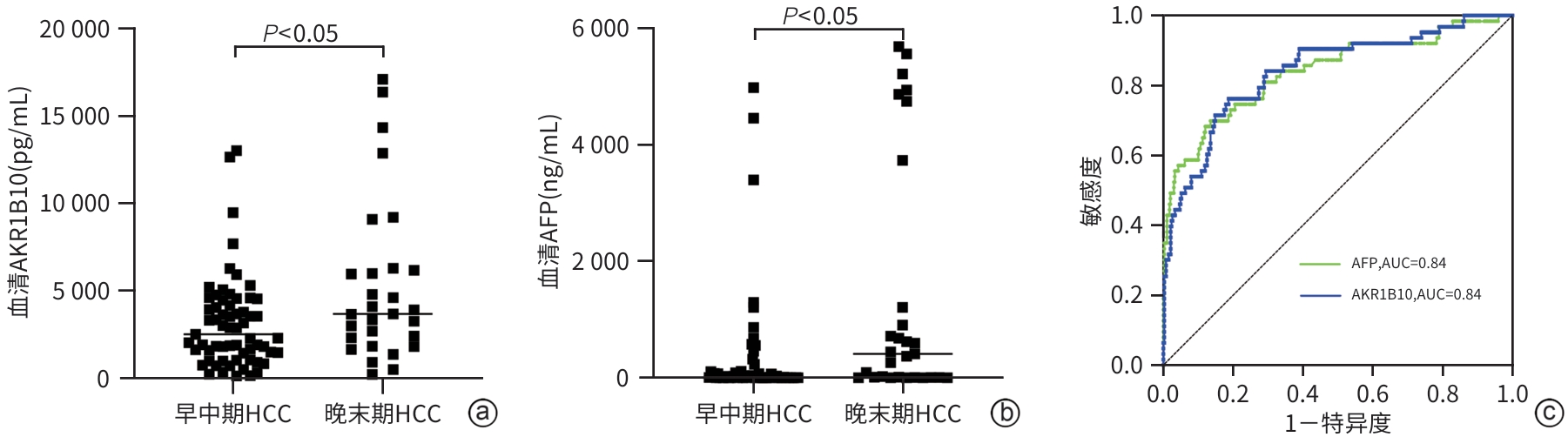
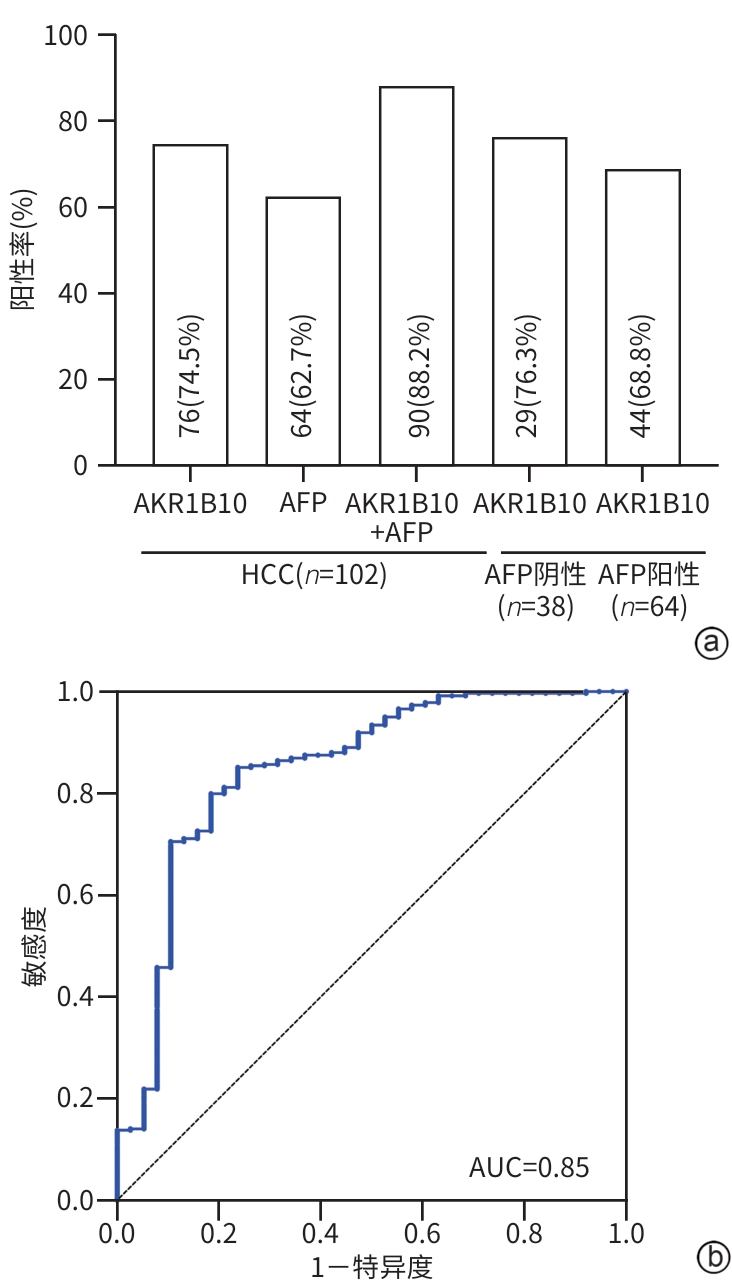
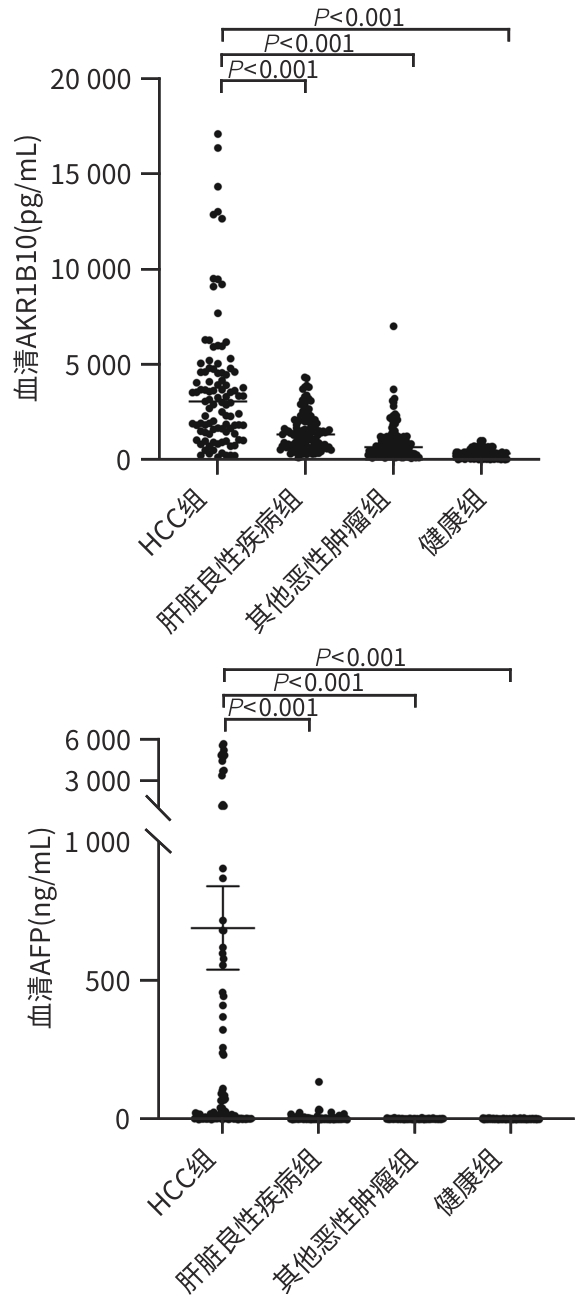
目的 通过分析血清醛酮还原酶家族1成员B10(AKR1B10)在肝细胞癌(HCC)患者中的表达情况,为HCC的临床诊断提供新的有价值的血清学标志物。 方法 选取2020年5月—2024年5月承德医学院附属医院就诊的102例HCC患者、119例肝脏良性疾病患者、132例其他恶性肿瘤患者和148例健康体检者为研究对象。通过酶联免疫吸附法和化学发光法分别检测血清AKR1B10和AFP水平。非正态分布的计量资料两组间比较采用Mann-Whitney U检验,Kruskal-Wallis H检验用于多组间的比较和进一步两两比较,计数资料组间比较采用χ2检验,利用受试者操作特征曲线(ROC曲线)下面积(AUC)评估检验效能。 结果 四组间AKR1B10表达差异有统计学意义[HCC组:3 053.79(1 475.67~4 605.86) pg/mL,肝脏良性疾病组:1 324.42(659.68~2 023.88) pg/mL,其他恶性肿瘤组:660.68(377.56~2 087.77) pg/mL,健康组:318.30(82.73~478.82) pg/mL,H=240.86, P<0.001)。进一步两两比较,HCC组明显高于肝脏良性疾病组、其他恶性肿瘤组和健康组(P值均<0.001)。HCC组与其他三组的ROC曲线结果显示,诊断HCC的最佳血清AKR1B10浓度界值为1 584.97 pg/mL,AUC为0.86,95%CI:0.82~0.90,敏感度为74.3%,特异度为85.2%。相较于单一指标,AKR1B10与AFP联合应用,可以提高HCC诊断的敏感度(81.8%)和特异度(91.4%)。AKR1B10诊断早中期HCC患者的AUC为0.84,95%CI:0.78~0.90,敏感度为76.2%,特异度为81.2%。AKR1B10诊断AFP阴性HCC患者的AUC为0.85,95%CI:0.77~0.92,敏感度为81.6%,特异度为79.9%。 结论 AKR1B10是一种有潜力的诊断HCC的新型血清学标志物,与AFP联合使用,可能提高HCC(尤其是早中期HCC和AFP阴性HCC)患者的检出率。 -
关键词:
- 癌, 肝细胞 /
- 醛酮还原酶家族1成员B10 /
- 甲胎蛋白类
Abstract:Objective To investigate the expression of serum Aldo-keto reductase family 1 member B10 (AKR1B10) in patients with hepatocellular carcinoma (HCC) in northern China, and to provide a new and valuable biomarker for the clinical diagnosis of HCC. Methods This study was conducted among 102 patients with HCC, 119 patients with benign liver disease, and 132 patients with other malignant tumors who attended The Affiliated Hospital of Chengde Medical University and 148 healthy individuals who underwent physical examination from May 2020 to May 2024. ELISA and chemiluminescence were used to measure the serum levels of AKR1B10 and alpha-fetoprotein (AFP). The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between two groups, and the Kruskal-Wallis H test was used for comparison between three groups and further comparison between two groups; the chi-square test was used for comparison of categorical data between groups. The area under the ROC curve (AUC) was used to assess diagnostic efficiency. Results The expression level of AKR1B10 was 3 053.79 (1 475.67 — 4 605.86) pg/mL in the HCC group, 1 324.42 (659.68 — 2 023.88) pg/mL in the benign liver disease group, 660.68 (377.56 — 2 087.77) pg/mL in the other malignant tumor group, and 318.30 (82.73 — 478.82) pg/mL in the healthy group, with a significant difference between the four groups (H=240.86, P<0.001), and further comparison between two groups showed that the HCC group had a significantly higher level than the other three groups (all P<0.001). The ROC curve analysis of the HCC group and the other three groups showed that serum AKR1B10 had an optimal cut-off value of 1 584.97 pg/mL in the diagnosis of HCC, with an AUC of 0.86 (95% confidence interval [CI]: 0.82 — 0.90), a sensitivity of 74.3%, and a specificity of 85.2%. Compared with each indicator alone, a combination of AKR1B10 and AFP could improve the sensitivity (81.8%) and specificity (91.4%) of HCC diagnosis. AKR1B10 had an AUC of 0.84 (95%CI: 0.78 — 0.90) in the diagnosis of patients with early- or middle-stage HCC, with a sensitivity of 76.2% and a specificity of 81.2%. AKR1B10 had an AUC of 0.85 (95%CI: 0.77 — 0.92) in the diagnosis of patients with AFP-negative HCC, with a sensitivity of 81.6% and a specificity of 79.9%. Conclusion AKR1B10 is a promising serological marker for the diagnosis of HCC, and a combination of AKR1B10 and AFP can improve the detection rate of HCC patients in northern China, especially those with early- or middle-stage HCC and AFP-negative HCC. -
表 1 一般资料比较
Table 1. Comparison of general information
项目 HCC组
(n=102)
健康组
(n=148)
肝脏良性疾病组(n=119) 其他恶性肿瘤组(n=132) 统计值 P值 男性[例(%)] 82(80.39) 82(55.40)1) 71(59.66)1) 73(55.30)1) χ2=20.0 <0.001 年龄(岁) 60(55~66) 45(35~55)1) 52(47~59)1) 61(54~66) H=112.4 <0.001 AFP(ng/mL) 24.4(4.1~458.0) 2.3(1.8~3.1)1) 2.8(2.0~3.5)1) 2.6(1.9~3.5)1) H=132.3 <0.001 CEA(ng/mL) 2.6(1.3~3.8) 1.6(0.9~2.6)1) 2.6(1.8~4.5) 2.1(1.3~4.7) H=33.2 <0.001 CA19-9(U/mL) 20.3(10.2~49.8) 6.6(5.2~14.4)1) 7.7(5.3~11.3)1) 16.2(7.4~50.8) H=105.8 <0.001 ALT(U/L) 27(18~43) 18(13~35)1) 22(15~35)1) 16(11~26)1) H=44.1 <0.001 AST(U/L) 38(26~76) 21(18~23)1) 25(19~63)1) 19(14~28)1) H=102.9 <0.001 GGT(U/L) 92(37~188) 23(16~37)1) 29(17~42)1) 32(17~957)1) H=94.6 <0.001 ALP(U/L) 145(115~261) 69(59~80)1) 105(90~131)1) 96(76~144)1) H=105.1 <0.001 LDH(U/L) 216(185~260) 160(148~180)1) 169(145~208)1) 176(156~201)1) H=78.7 <0.001 TBil(μmol/L) 17.8(11.9~26.2) 14.0(11.5~17.3)1) 15.0(11.1~23.3) 12.0(8.1~15.5)1) H=44.9 <0.001 DBil(μmol/L) 7.4(4.7~11.5) 3.1(2.5~3.7)1) 5.4(3.7~9.1)1) 3(2.3~5.2)1) H=164.2 <0.001 TP(g/L) 69(66~74) 72(70~74)1) 72(67~75)1) 67(62~71)1) H=60.9 <0.001 Alb(g/L) 42(36~46) 45(43~47)1) 47(37~49)1) 41(39~44) H=56.2 <0.001 PA(mg/L) 167(102~208) 274(230~316)1) 207(149~259)1) 225(183~267)1) H=112.7 <0.001 注:与HCC组比较,1)P<0.05。
-
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] LIU YX, ZHENG ZB. Understanding the global cancer statistics 2022: Growing cancer burden[J]. Sci China Life Sci, 2024, 67( 10): 2274- 2276. DOI: 10.1007/s11427-024-2657-y. [3] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [4] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [5] LU J, ZHANG XP, ZHONG BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: Comparing east and west[J]. Lancet Gastroenterol Hepatol, 2019, 4( 9): 721- 730. DOI: 10.1016/S2468-1253(19)30178-5. [6] DING CM, HOU JF, TAO GW, et al. Early diagnosis and screening of hepatocellular carcinoma[J/OL]. Chin J Hepat Surg(Electronic Edition), 2023, 12( 1): 22- 28. DOI: 10.3877/cma.j.issn.2095-3232.2023.01.005.丁成明, 侯嘉丰, 陶光伟, 等. 肝细胞癌早期诊断和筛查[J/OL]. 中华肝脏外科手术学电子杂志, 2023, 12( 1): 22- 28. DOI: 10.3877/cma.j.issn.2095-3232.2023.01.005. [7] WANG XP, WANG QX. Alpha-fetoprotein and hepatocellular carcinoma immunity[J]. Can J Gastroenterol Hepatol, 2018, 2018: 9049252. DOI: 10.1155/2018/9049252. [8] LU QQ, LI J, CAO H, et al. Comparison of diagnostic accuracy of Midkine and AFP for detecting hepatocellular carcinoma: A systematic review and meta-analysis[J]. Biosci Rep, 2020, 40( 3): BSR20192424. DOI: 10.1042/BSR20192424. [9] YU XP, YANG RY, HE ZM, et al. Establishment and validation of nomogram of cancer specific survival of patients with hepatocellular carcinoma with negative alpha fetoprotein based on SEER Database[J]. J Jilin Univ(Med Edit), 2024, 50( 1): 188- 197. DOI: 10.13481/j.1671-587X.20240123.余孝鹏, 杨仁义, 贺佐梅, 等. 基于SEER数据库建立和验证甲胎蛋白阴性肝细胞癌患者癌症特异生存期的列线图[J]. 吉林大学学报(医学版), 2024, 50( 1): 188- 197. DOI: 10.13481/j.1671-587X.20240123. [10] LIU YZ, ZHANG J, LIU H, et al. Compensatory upregulation of aldo-keto reductase 1B10 to protect hepatocytes against oxidative stress during hepatocarcinogenesis[J]. Am J Cancer Res, 2019, 9( 12): 2730- 2748. [11] SINGAL AG, LLOVET JM, YARCHOAN M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma[J]. Hepatology, 2023, 78( 6): 1922- 1965. DOI: 10.1097/hep.0000000000000466. [12] FORNER A, REIG M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2018, 391( 10127): 1301- 1314. DOI: 10.1016/s0140-6736(18)30010-2. [13] MORIGUCHI M, KATAOKA S, ITOH Y. Evolution of systemic treatment for hepatocellular carcinoma: Changing treatment strategies and concepts[J]. Cancers(Basel), 2024, 16( 13): 2387. DOI: 10.3390/cancers16132387. [14] YUE YX, NING SK, LIU JB, et al. Comparative analysis of the efficacy of interventional therapy and interventional therapy combined with targeted drugs for advanced primary liver cancer[J]. Chin J Cancer Prev Treat, 2021, 28( 10): 788- 791. DOI: 10.16073/j.cnki.cjcpt.2021.10.13.岳衍晓, 宁尚昆, 刘吉兵, 等. 晚期原发性肝癌介入治疗与联合靶向药物治疗效果对比分析[J]. 中华肿瘤防治杂志, 2021, 28( 10): 788- 791. DOI: 10.16073/j.cnki.cjcpt.2021.10.13. [15] FU YL, SHI WX, HUANG S, et al. Comparison of prognostic evaluation and therapeutic guidance value of three clinical staging systems for hepatocellular carcinoma[J]. J Guangxi Med Univ, 2018, 35( 12): 1706- 1709. DOI: 10.16190/j.cnki.45-1211/r.2018.12.025.付艳龙, 石文新, 黄山, 等. 肝细胞癌3种临床分期系统预后评估和治疗指导价值的比较[J]. 广西医科大学学报, 2018, 35( 12): 1706- 1709. DOI: 10.16190/j.cnki.45-1211/r.2018.12.025. [16] YE X, LI CY, ZU XY, et al. A large-scale multicenter study validates aldo-keto reductase family 1 member B10 as a prevalent serum marker for detection of hepatocellular carcinoma[J]. Hepatology, 2019, 69( 6): 2489- 2501. DOI: 10.1002/hep.30519. [17] HUNG JJ, YEH YC, HSU WH. Prognostic significance of AKR1B10 in patients with resected lung adenocarcinoma[J]. Thorac Cancer, 2018, 9( 11): 1492- 1499. DOI: 10.1111/1759-7714.12863. [18] ENDO S, MATSUNAGA T, NISHINAKA T. The role of AKR1B10 in physiology and pathophysiology[J]. Metabolites, 2021, 11( 6): 332. DOI: 10.3390/metabo11060332. [19] SHI J, CHEN LX, CHEN Y, et al. Aldo-Keto Reductase Family 1 Member B10(AKR1B10) overexpression in tumors predicts worse overall survival in hepatocellular carcinoma[J]. J Cancer, 2019, 10( 20): 4892- 4901. DOI: 10.7150/jca.32768. [20] QU JY, LI J, ZHANG YM, et al. AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-κB signaling pathway[J]. Cell Biosci, 2021, 11( 1): 163. DOI: 10.1186/s13578-021-00677-3. [21] TREVISANI F, GARUTI F, NERI A. Alpha-fetoprotein for diagnosis, prognosis, and transplant selection[J]. Semin Liver Dis, 2019, 39( 2): 163- 177. DOI: 10.1055/s-0039-1677768. [22] EDOO MIA, CHUTTURGHOON VK, WUSU-ANSAH GK, et al. Serum biomarkers AFP, CEA and CA19-9 combined detection for early diagnosis of hepatocellular carcinoma[J]. Iran J Public Health, 2019, 48( 2): 314- 322. [23] WANG Y, XU WL, CHAI XZ. Application of combined serum AFP and AKR1B10 in diagnosis of hepatocellular carcinoma[J]. Chin J Integr Tradit West Med Liver Dis, 2020, 30( 6): 541- 543. DOI: 10.3969/j.issn.1005-0264.2020.06.002.王月, 许炜璐, 柴晓哲. 血清AKR1B10和甲胎蛋白联合检测对肝细胞癌诊断的意义[J]. 中西医结合肝病杂志, 2020, 30( 6): 541- 543. DOI: 10.3969/j.issn.1005-0264.2020.06.002. [24] ZHU RP, XIAO J, LUO DT, et al. Serum AKR1B10 predicts the risk of hepatocellular carcinoma—A retrospective single-center study[J]. Gastroenterol Hepatol, 2019, 42( 10): 614- 621. DOI: 10.1016/j.gastrohep.2019.06.007. -



 PDF下载 ( 1788 KB)
PDF下载 ( 1788 KB)

 下载:
下载: