FibroScan、GPR评分、S指数、IL-6及TNF-α对HBeAg阳性慢性乙型肝炎肝纤维化的诊断价值
DOI: 10.12449/JCH250411
Value of FibroScan, gamma-glutamyl transpeptidase-to-platelet ratio, S index, interleukin-6, and tumor necrosis factor-α in the diagnosis of HBeAg-positive chronic hepatitis B liver fibrosis
-
摘要:
目的 本研究通过分析无创影像学检测(FibroScan)、两种血清学模型(GPR评分、S指数)和两种炎性因子(IL-6、TNF-α)预测HBeAg阳性慢性乙型肝炎(CHB)患者肝纤维化的价值,以及对肝活检病理分期一致性评估,为CHB的早期干预提供预警。 方法 回顾性选取2019年1月—2023年12月在昆明市第三人民医院行肝穿刺活检的131例HBeAg阳性CHB患者为研究对象。收集患者肝活检结果,肝活检前完善相关检查:TBil、ALT、PLT、GGT、Alb、IL-6、TNF-α及肝硬度检测(LSM)、腹部超声。符合正态分布的计量资料多组间比较采用方差分析;非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验。计数资料组间比较采用χ2检验。采用Kappa检验分析LSM无创组织学分期与肝活检病理分期的一致性;单一变量与FibroScan诊断肝纤维化分期的相关性采用Spearman分析。采用Logistic回归分析构建联合预测因子。采用受试者操作特征曲线(ROC曲线)分析单项指标以及联合预测模型对肝纤维化的诊断价值,采用Delong检验比较各ROC曲线下面积间的差异。 结果 在肝脏病理活检炎症程度的一致性检查中Kappa值为0.807(P<0.001),肝脏病理活检肝纤维化程度的一致性检查中Kappa值为0.827(P<0.001),表明FibroScan无创组织学分期与肝活检病理在炎症分期和肝纤维化分期中均有良好一致性。年龄与LSM、GPR评分、S指数、IL-6、TNF-α均呈正相关(P值均<0.05)。GPR评分、S指数、IL-6、TNF-α与LSM均呈正相关(P值均<0.05)。GPR评分、S指数、IL-6、TNF-α均为诊断显著期肝纤维化(≥S2)和进展期肝纤维化(≥S3)的独立危险因素(P值均<0.05)。单独预测显著期肝纤维化(≥S2)的诊断价值从高到低依次为GPR评分、S指数、IL-6和TNF-α,单独预测进展期肝纤维化(≥S3)的诊断价值从高到低依次为S指数、GPR评分、TNF-α和IL-6。而联合模型的预测价值高于单项指标的预测价值(P值均<0.05)。 结论 FibroScan的无创组织学分期与肝活检病理分期的一致性良好。GPR评分、S指数、IL-6、TNF-α是评价CHB不同程度纤维化的独立危险因素,上述指标所建立的联合预测模型能更好地诊断肝纤维化。 Abstract:Objective To investigate the value of noninvasive imaging detection (FibroScan), two serological models of gamma-glutamyl transpeptidase-to-platelet ratio (GPR) score and S index, and two inflammatory factors of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in predicting liver fibrosis in patients with HBeAg-positive chronic hepatitis B (CHB), as well as the consistency of liver biopsy in pathological staging, and to provide early warning for early intervention of CHB. Methods A retrospective analysis was performed for 131 HBeAg-positive CHB patients who underwent liver biopsy in The Third People’s Hospital of Kunming from January 2019 to December 2023. The results of liver biopsy were collected from all patients, and related examinations were performed before liver biopsy, including total bilirubin, alanine aminotransferase, platelet count, gamma-glutamyl transpeptidase, albumin, IL-6, TNF-α, liver stiffness measurement (LSM), and abdominal ultrasound. An analysis of variance was used for comparison of normally distributed continuous data between groups, and the Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A Kappa analysis was used to investigate the consistency between LSM noninvasive histological staging and pathological staging based on liver biopsy, and the Spearman analysis was used to investigate the correlation between each variable and FibroScan in the diagnosis of liver fibrosis stage. The Logistic regression analysis was used to construct joint predictive factors. The receiver operating characteristic (ROC) curve was used to evaluate the value of each indicator alone and the joint predictive model in the diagnosis of liver fibrosis, and the Delong test was used for comparison of the area under the ROC curve (AUC). Results In the consistency check, inflammation degree based on liver biopsy had a Kappa value of 0.807 (P<0.001), and liver fibrosis degree based on liver biopsy had a Kappa value of 0.827 (P<0.001), suggesting that FibroScan noninvasive histological staging and liver biopsy showed good consistency in assessing inflammation degree and liver fibrosis stage. Age was positively correlated with LSM, GPR score, S index, IL-6, and TNF-α (all P<0.05), and GPR score, S index, IL-6, and TNF-α were positively correlated with LSM (all P<0.05). GPR score, S index, IL-6, and TNF-α were all independent risk factors for diagnosing significant liver fibrosis (≥S2) and progressive liver fibrosis (≥S3) (all P<0.05). As for each indicator alone, GPR score had the highest value in the diagnosis of significant liver fibrosis (≥S2), followed by S index, IL-6, and TNF-α, while S index had the highest value in the diagnosis of progressive liver fibrosis (≥S3), followed by GPR score, TNF-α, and IL-6. The joint model had a higher predictive value than each indicator alone (all P<0.05). Conclusion There is a good consistency between FibroScan noninvasive histological staging and pathological staging based on liver biopsy. GPR score, S index, IL-6, and TNF-α are independent risk factors for evaluating different degree of liver fibrosis in CHB, and the combined prediction model established by them can better diagnose liver fibrosis. -
Key words:
- Hepatitis B, Chronic /
- Hepatic Fibrosis /
- Diagnosis
-
表 1 一般临床资料分析
Table 1. General clinical data analysis
临床特征 S0~S1(n=58) S2(n=22) S3~S4(n=51) 统计值 P值 性别[例(%)] χ2=0.014 0.993 男 37(63.79) 14(63.36) 32(62.75) 女 21(36.21) 8(36.21) 19(37.25) 年龄(岁) 35.36±10.60 37.36±12.00 43.12±14.12 F=5.552 0.005 LSM(kPa) 6.50(5.20~7.00) 9.80(9.28~11.53) 16.70(14.50~24.50) H=108.710 <0.001 BMI(kg/m2) 23.40(20.60~25.40) 22.50(20.33~24.50) 21.60(19.50~23.60) H=3.590 0.166 TBil(μmol/L) 6.50(5.20~7.00) 23.00(16.98~52.65) 24.60(18.40~35.60) H=0.948 0.622 ALT(U/L) 120.0(81.75~143.00) 102.00(74.00~112.25) 125.00(93.00~146.00) H=5.552 0.062 腹水[例(%)] 0(0.0) 0(0.0) 5(9.8) χ2=8.154 0.017 PLT(×109/L) 265.00(241.00~315.00) 164.50(143.25~172.00) 135.00(103.00~165.00) H=56.742 <0.001 Alb(g/L) 41.00(38.75~46.25) 39.50(37.75~41.25) 32.00(28.00~35.00) H=74.563 <0.001 GGT(U/L) 61.00(37.75~107.50) 85.00(67.50~125.25) 125.00(85.00~254.00) H=30.549 <0.001 GPR评分(分) 0.44(0.27~0.76) 1.12(0.81~1.95) 1.93(1.15~3.01) H=72.946 <0.001 S指数 0.18(0.09~0.30) 0.56(0.30~1.07) 1.30(0.50~1.92) H=71.431 <0.001 IL-6(pg/mL) 6.35(3.58~12.60) 12.53(10.31~16.58) 13.60(11.99~16.93) H=44.157 <0.001 TNF-α(pg/mL) 4.85(3.52~12.95) 10.57(6.86~14.63) 16.47(12.58~17.84) H=40.600 <0.001 表 2 FibroScan与肝脏病理活检炎症程度的一致性检查
Table 2. Consistency check between FibroScan and inflammation degree in liver pathological biopsy
项目 F0(例) F1(例) F2(例) F3(例) F4(例) 合计(例) G0(例) 18 0 0 0 0 18 G1(例) 5 31 2 0 0 38 G2(例) 0 3 18 1 0 22 G3(例) 0 0 3 22 3 28 G4(例) 0 0 0 3 22 25 合计(例) 23 34 23 26 25 131 Kappa值 0.807 U值 18.276 P值 <0.001 表 3 FibroScan与肝脏病理活检肝纤维化程度的一致性检查
Table 3. Consistency check between Fibroscan and liver pathological biopsy for assessing the degree of liver fibrosis
项目 F0(例) F1(例) F2(例) F3(例) F4(例) 合计(例) S0(例) 20 3 1 0 0 24 S1(例) 3 29 2 0 0 34 S2(例) 0 0 19 3 0 22 S3(例) 0 0 2 22 2 26 S4(例) 0 0 0 2 23 25 合计(例) 23 32 24 27 25 131 Kappa值 0.827 U值 18.831 P值 <0.001 表 4 LSM、GPR评分、S指数、IL-6、TNF-α与年龄、BMI、TBil、ALT的相关性分析
Table 4. Correlation analysis of LSM, GPR score, S index, IL6, TNF-α with age, BMI, TBil, ALT
指标 LSM GPR评分 S指数 IL-6 TNF-α 年龄 r值 0.214 0.207 0.175 0.240 0.193 P值 0.014 0.018 0.046 0.006 0.027 BMI r值 -0.104 0.038 -0.039 -0.095 -0.095 P值 0.236 0.666 0.658 0.282 0.281 TBil r值 0.097 0.025 0.065 0.056 0.024 P值 0.270 0.773 0.461 0.525 0.785 ALT r值 0.138 0.061 0.130 0.007 0.064 P值 0.116 0.490 0.140 0.936 0.469 表 5 GPR评分、S指数、IL-6、TNF-α与LSM的相关性分析
Table 5. Correlation analysis between GPR score, S index, IL-6, TNF-α and LSM
指标 r值 P值 GPR评分 0.669 <0.001 S指数 0.762 <0.001 IL-6 0.543 <0.001 TNF-α 0.511 <0.001 表 6 HBeAg阳性CHB患者显著期肝纤维化和进展期肝纤维化的多因素二元Logistic回归分析
Table 6. Multivariate binary Logistic regression analysis of significant and advanced liver fibrosis in HBeAg positive CHB patients
指标 β值 SE Wald P值 OR 95%CI 显著期肝纤维化(≥S2) GPR评分 3.180 0.966 10.203 0.001 24.057 3.418~169.344 S指数 2.826 1.107 6.519 0.011 16.881 1.928~147.763 IL-6 0.200 0.075 7.037 0.008 1.221 1.054~1.415 TNF-α 0.175 0.068 6.532 0.011 1.191 1.042~1.362 进展期肝纤维化(≥S3) GPR评分 0.883 0.288 9.392 0.002 2.418 1.375~4.253 S指数 0.999 0.405 6.074 0.014 2.715 1.227~6.006 IL-6 0.205 0.072 8.168 0.004 1.227 1.066~1.412 TNF-α 0.233 0.071 10.714 0.001 1.263 1.098~1.452 表 7 无创指标预测肝纤维化效能分析
Table 7. Analysis of non-invasive indicators for predicting the efficacy of liver fibrosis
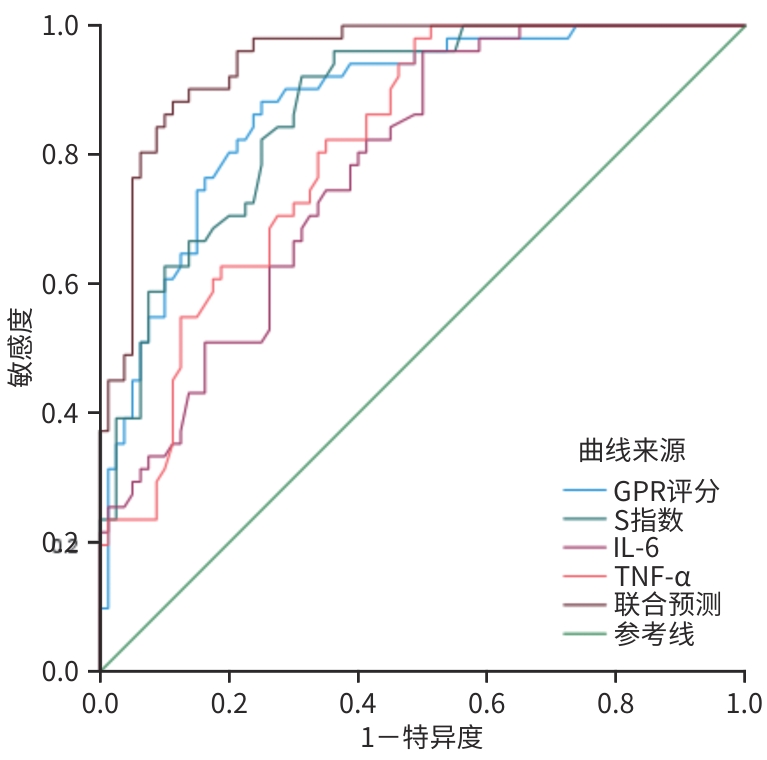
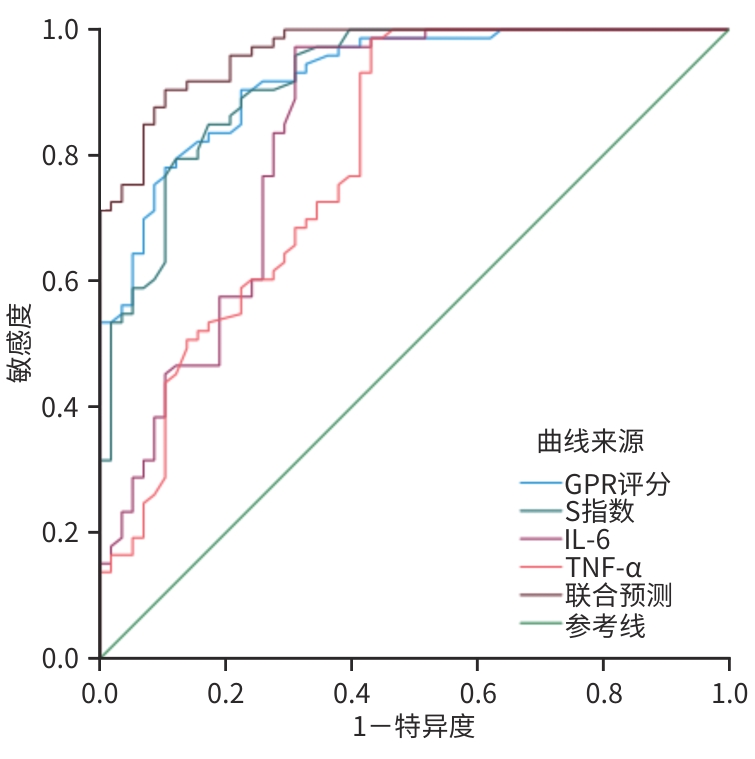
指标 AUC 截断值 约登指数 敏感度(%) 特异度(%) 95%CI Z值1) P值1) 显著期肝纤维化(≥S2) GPR评分 0.924 0.78 0.680 90.4 77.6 0.882~0.967 -3.243 0.001 S指数 0.920 0.47 0.674 79.5 87.9 0.875~0.965 -2.878 0.004 IL-6 0.835 8.46 0.663 96.3 69.0 0.761~0.908 -4.067 0.001 TNF-α 0.795 6.21 0.555 98.6 56.9 0.716~0.874 -4.695 0.001 联合预测 0.979 0.873 95.9 91.4 0.961~0.998 进展期肝纤维化(≥S3) GPR评分 0.877 0.93 0.632 88.2 75.0 0.818~0.936 -2.817 0.005 S指数 0.944 0.40 0.609 92.2 68.7 0.907~0.980 -3.070 0.002 IL-6 0.779 8.46 0.461 96.1 50.0 0.703~0.856 -0.572 0.001 TNF-α 0.811 7.98 0.492 98.0 51.2 0.740~0.882 -4.453 0.001 联合预测 0.947 0.763 86.3 90.0 0.896~0.976 注:1)与联合预测的AUC比较。
-
[1] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Infect Dis Info, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 传染病信息, 2023, 36( 1): 1- 17. DOI: 10.3969/j.issn.1007-8134.2023.01.01. [2] DONG BT, HUANG S, LYU GR, et al. Assessment of liver fibrosis with liver and spleen stiffness measured by sound touch elastography, serum fibrosis markers in patients with chronic hepatitis B[J]. J Dig Dis, 2021, 22( 6): 342- 350. DOI: 10.1111/1751-2980.12991. [3] ZHU L, YANG JR, HE LL, et al. Advances on the application of transient elastography in the diagnosis of liver fibrosis[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 3): 16- 22. DOI: 10.3969/j.issn.1674-7380.2023.03.003.朱璐, 杨君茹, 何玲玲, 等. 瞬时弹性成像在肝纤维化诊断中的应用研究进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 3): 16- 22. DOI: 10.3969/j.issn.1674-7380.2023.03.003. [4] Chinese Foundation for Hepatitis Prevention and Control; Chinese Society of Infectious Disease and Chinese Society of Hepatology, Chinese Medical Association; Liver Disease Committee of Chinese Research Hospital Association. Consensus on clinical application of transient elastography detecting liver fibrosis: A 2018 update[J]. Chin J Hepatol, 2019, 27( 3): 182- 191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004.中国肝炎防治基金会, 中华医学会感染病学分会, 中华医学会肝病学分会和中国研究型医院学会肝病专业委员会. 瞬时弹性成像技术诊断肝纤维化专家共识(2018年更新版)[J]. 中华肝脏病杂志, 2019, 27( 3): 182- 191. DOI: 10.3760/cma.j.issn.1007-3418.2019.03.004. [5] Chinese Medical Association Hepatology Branch, Gastroenterology Branch of Chinese Medical Association, Consensus on the diagnosis and therapy of hepatic fibrosis( 2019)[J]. J Clin Hepatol, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007.中华医学会肝病学分会, 中华医学会消化病学分会, 中华医学会感染病学分会. 肝纤维化诊断及治疗共识(2019年)[J]. 临床肝胆病杂志, 2019, 35( 10): 2163- 2172. DOI: 10.3969/j.issn.1001-5256.2019.10.007. [6] TANG QR, LAI CX, WANG F, et al. Establishment of a noninvasive diagnostic model for chronic hepatitis B liver fibrosis patients with normal aminotransferases aged≤30 years[J]. J Clin Hepatol, 2024, 40( 9): 1790- 1795. DOI: 10.12449/JCH240912.唐情容, 赖长祥, 王方, 等. 年龄≤30岁转氨酶正常的慢性乙型肝炎肝纤维化患者无创诊断模型的建立[J]. 临床肝胆病杂志, 2024, 40( 9): 1790- 1795. DOI: 10.12449/JCH240912. [7] HUANG BS, OU LX, ZHANG YJ, et al. Value of traditional noninvasive fibrosis models in diagnosis of significant liver fibrosis in patients with chronic hepatitis B and metabolic associated fatty liver disease[J]. J Clin Hepatol, 2023, 39( 9): 2110- 2116. DOI: 10.3969/j.issn.1001-5256.2023.09.012.黄柏盛, 区蓝芯, 张莹洁, 等. 传统非侵入性纤维化模型对慢性乙型肝炎合并代谢相关脂肪性肝病发生显著肝纤维化的诊断价值[J]. 临床肝胆病杂志, 2023, 39( 9): 2110- 2116. DOI: 10.3969/j.issn.1001-5256.2023.09.012. [8] ASFUROGLU-KALKAN EA, SOYKAN I. Role of non-invasive scoring systems in detecting fibrosis in chronic hepatitis B[J]. Klimik Derg, 2022, 35( 3): 164- 170. DOI: 10.36519/kd.2022.4338. [9] HUANG K, LI QY, ZENG WM, et al. Ultrasound score combined with liver stiffness measurement by sound touch elastography for staging liver fibrosis in patients with chronic hepatitis B: A clinical prospective study[J]. Ann Transl Med, 2022, 10( 6): 271. DOI: 10.21037/atm-22-505. [10] HUANG LL, YU XP, LI JL, et al. Effect of liver inflammation on accuracy of FibroScan device in assessing liver fibrosis stage in patients with chronic hepatitis B virus infection[J]. World J Gastroenterol, 2021, 27( 7): 641- 653. DOI: 10.3748/wjg.v27.i7.641. [11] MYERS RP, POMIER-LAYRARGUES G, KIRSCH R, et al. Discordance in fibrosis staging between liver biopsy and transient elastography using the FibroScan XL probe[J]. J Hepatol, 2012, 56( 3): 564- 570. DOI: 10.1016/j.jhep.2011.10.007. [12] OEDA S, TANAKA K, OSHIMA A, et al. Diagnostic accuracy of FibroScan and factors affecting measurements[J]. Diagnostics(Basel), 2020, 10( 11): 940. DOI: 10.3390/diagnostics10110940. [13] GOYAL R, MALLICK SR, MAHANTA M, et al. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B[J]. J Gastroenterol Hepatol, 2013, 28( 11): 1738- 1745. DOI: 10.1111/jgh.12318. [14] LEMOINE M, SHIMAKAWA Y, NAYAGAM S, et al. The gamma-glutamyl transpeptidase to platelet ratio(GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa[J]. Gut, 2016, 65( 8): 1369- 1376. DOI: 10.1136/gutjnl-2015-309260. [15] LIANG CF, CHANG YN, PENG XR, et al. Analysis of liver pathological characteristics and exploration of noninvasive markers of liver fibrosis in children with chronic hepatitis B[J]. Chin J Hepatol, 2021, 29( 6): 551- 557. DOI: 10.3760/cma.j.cn501113-20210423-00197.梁程飞, 常宇南, 彭小蓉, 等. 慢性乙型肝炎儿童肝脏病理特征分析及其肝纤维化非侵入性指标探索[J]. 中华肝脏病杂志, 2021, 29( 6): 551- 557. DOI: 10.3760/cma.j.cn501113-20210423-00197. [16] WANG JX, SUN XC, WEI SB, et al. Noninvasive models for the prediction of liver fibrosis in patients with chronic hepatitis B[J]. BMC Gastroenterol, 2024, 24( 1): 183. DOI: 10.1186/s12876-024-03270-3. [17] LIU XQ, LI H, WEI L, et al. Optimized cutoffs of gamma-glutamyl transpeptidase-to-platelet ratio, aspartate aminotransferase-to-platelet ratio index, and fibrosis-4 scoring systems for exclusion of cirrhosis in patients with chronic hepatitis B[J]. Hepatol Commun, 2022, 6( 7): 1664- 1672. DOI: 10.1002/hep4.1938. [18] ZHOU K, ZHENG RD, XIAN JC, et al. Building a noninvasive diagnostic model based on conventional laboratory markers to predict liver fibrosis[J]. Chin Hepatol, 2008, 13( 5): 362- 367. DOI: 10.14000/j.cnki.issn.1008-1704.2008.05.004.周琨, 郑瑞丹, 咸建春, 等. 从常规指标中建立肝纤维化非创伤性诊断模型[J]. 肝脏, 2008, 13( 5): 362- 367. DOI: 10.14000/j.cnki.issn.1008-1704.2008.05.004. [19] XU B, SUN L. The diagnostic value of liver stiffness measurement combined with S index in the degree of hepatitis B liver fibrosis[J]. J Hainan Med Univ, 2023, 29( 10): 746- 750. DOI: 10.13210/j.cnki.jhmu.20230318.002.许斌, 孙龙. 肝硬度值联合S指数对乙型肝炎患者肝纤维化程度的诊断价值[J]. 海南医学院学报, 2023, 29( 10): 746- 750. DOI: 10.13210/j.cnki.jhmu.20230318.002. [20] EKIN N, UCMAK F, EBIK B, et al. GPR, King’s Score and S-Index are superior to other non-invasive fibrosis markers in predicting the liver fibrosis in chronic hepatitis B patients[J]. Acta Gastroenterol Belg, 2022, 85( 1): 62- 68. DOI: 10.51821/85.1.9156. [21] ZHANG K, ZHANG MX, MENG XX, et al. Targeting GPR65 alleviates hepatic inflammation and fibrosis by suppressing the JNK and NF-κB pathways[J]. Mil Med Res, 2023, 10( 1): 56. DOI: 10.1186/s40779-023-00494-4. [22] ELNFARAWY AA, NASHY AE, ABOZAID AM, et al. Vinpocetine attenuates thioacetamide-induced liver fibrosis in rats[J]. Hum Exp Toxicol, 2021, 40( 2): 355- 368. DOI: 10.1177/0960327120947453. [23] CHEN CC, CHEN CY, YEH CT, et al. Corylin attenuates CCl4-induced liver fibrosis in mice by regulating the GAS6/AXL signaling pathway in hepatic stellate cells[J]. Int J Mol Sci, 2023, 24( 23): 16936. DOI: 10.3390/ijms242316936. -



 PDF下载 ( 1195 KB)
PDF下载 ( 1195 KB)

 下载:
下载: