高脂高糖高胆固醇饮食联合四氯化碳诱导的代谢相关脂肪性肝炎小鼠模型肝脏胆汁酸谱的变化
DOI: 10.12449/JCH250410
Changes in hepatic bile acid profile in a mouse model of metabolic-associated steatohepatitis induced by a high-fat, high-sugar, and high-cholesterol diet combined with carbon tetrachloride
-
摘要:
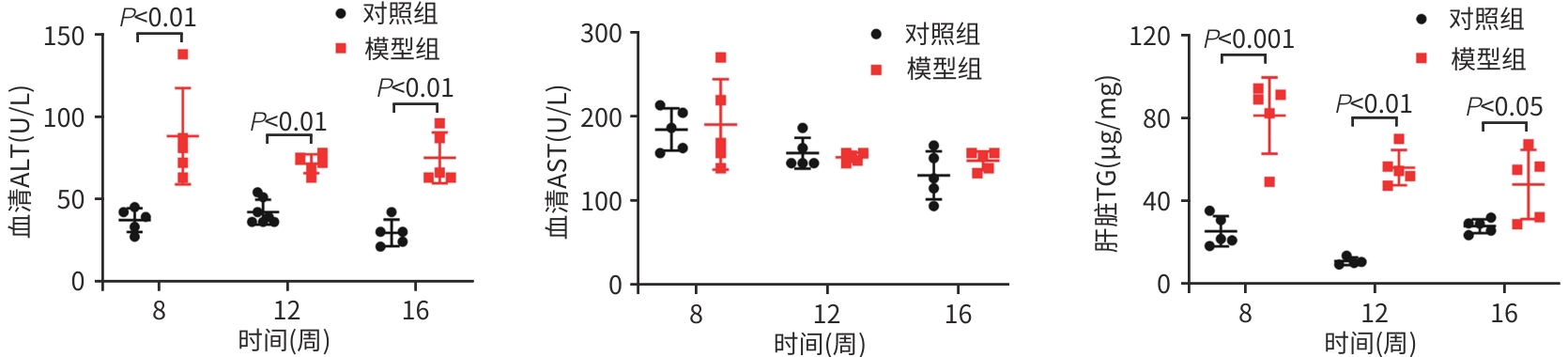
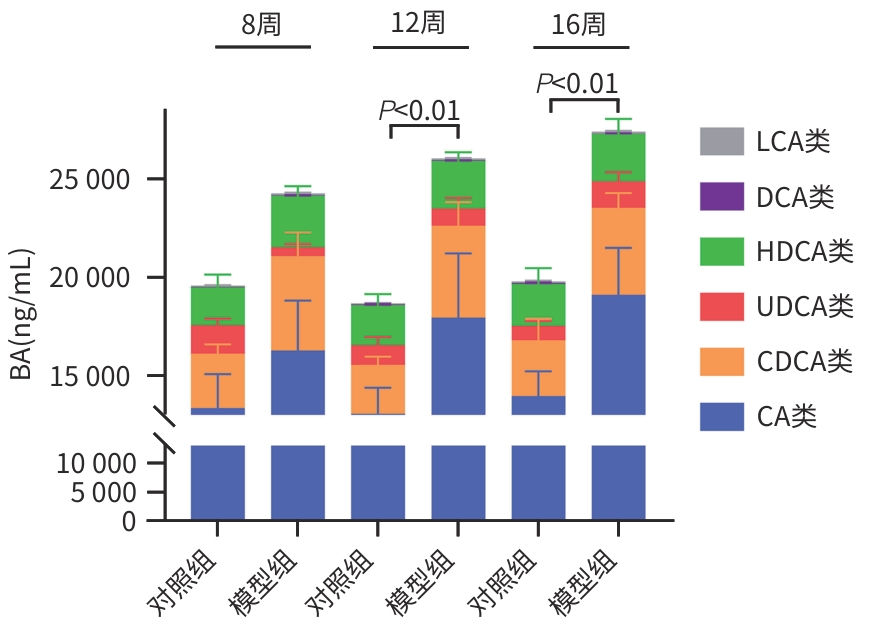
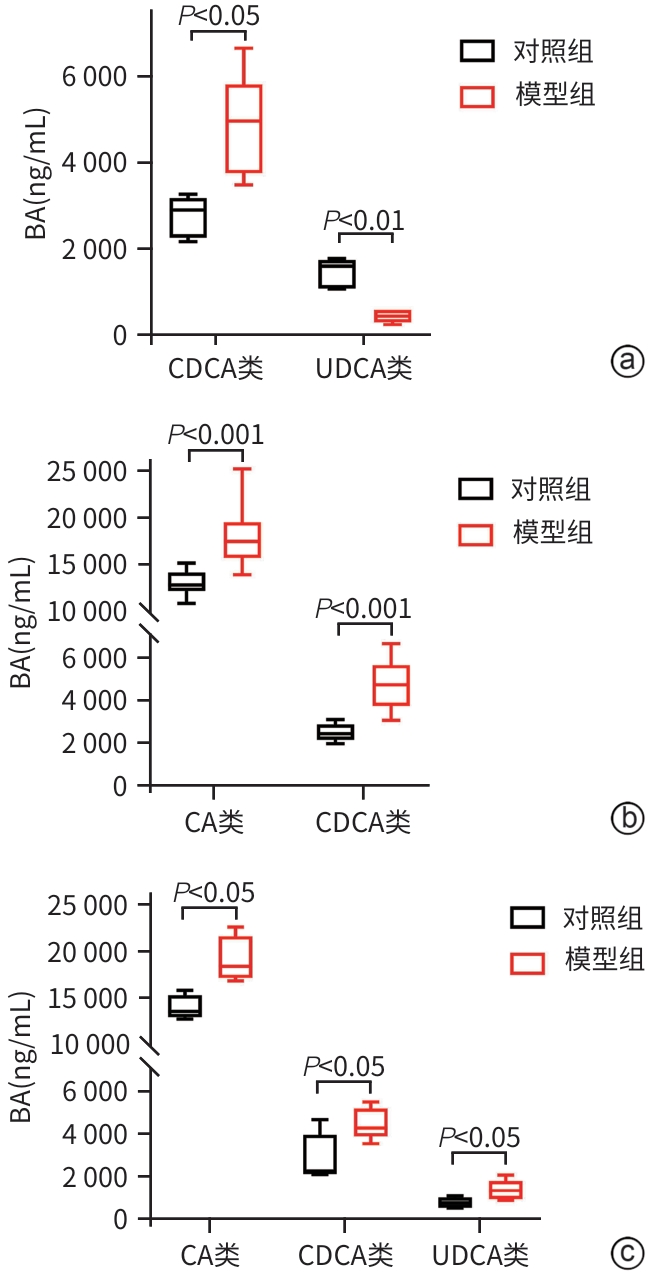
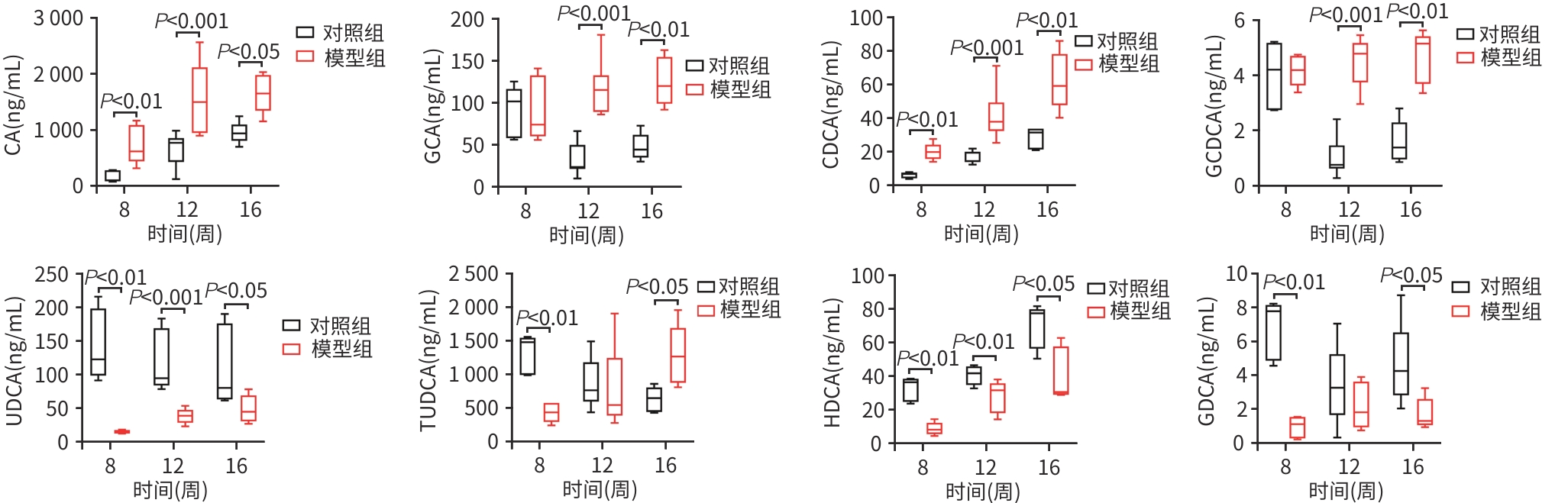
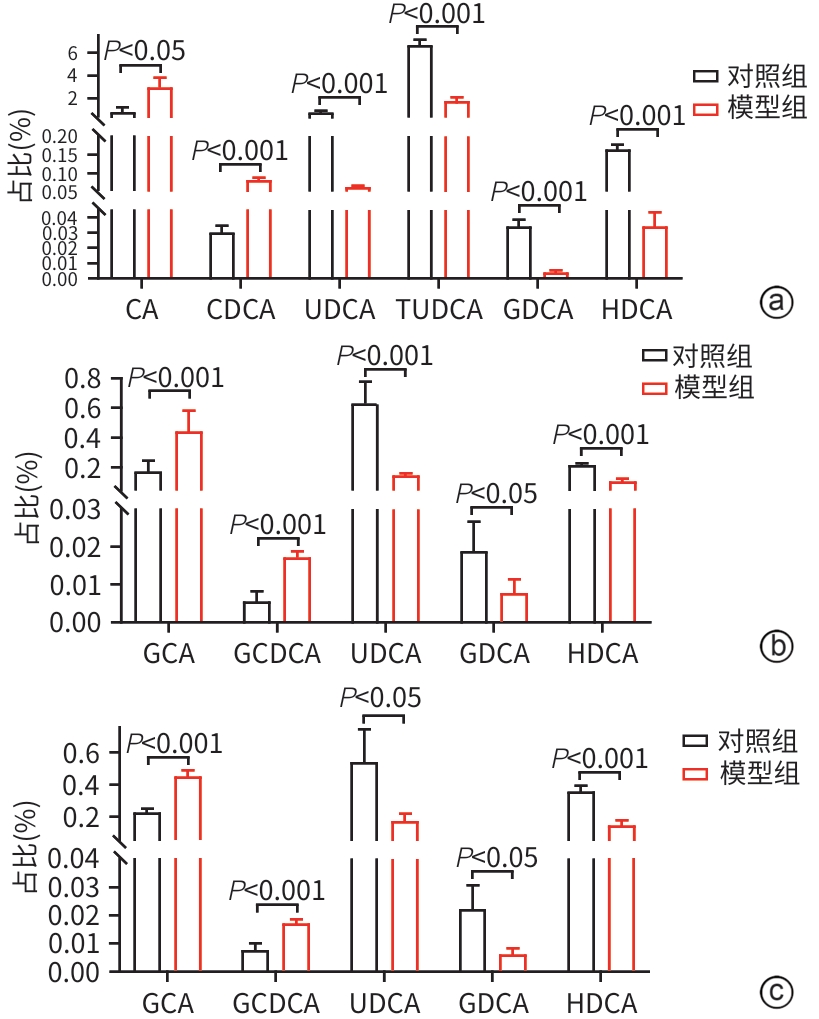
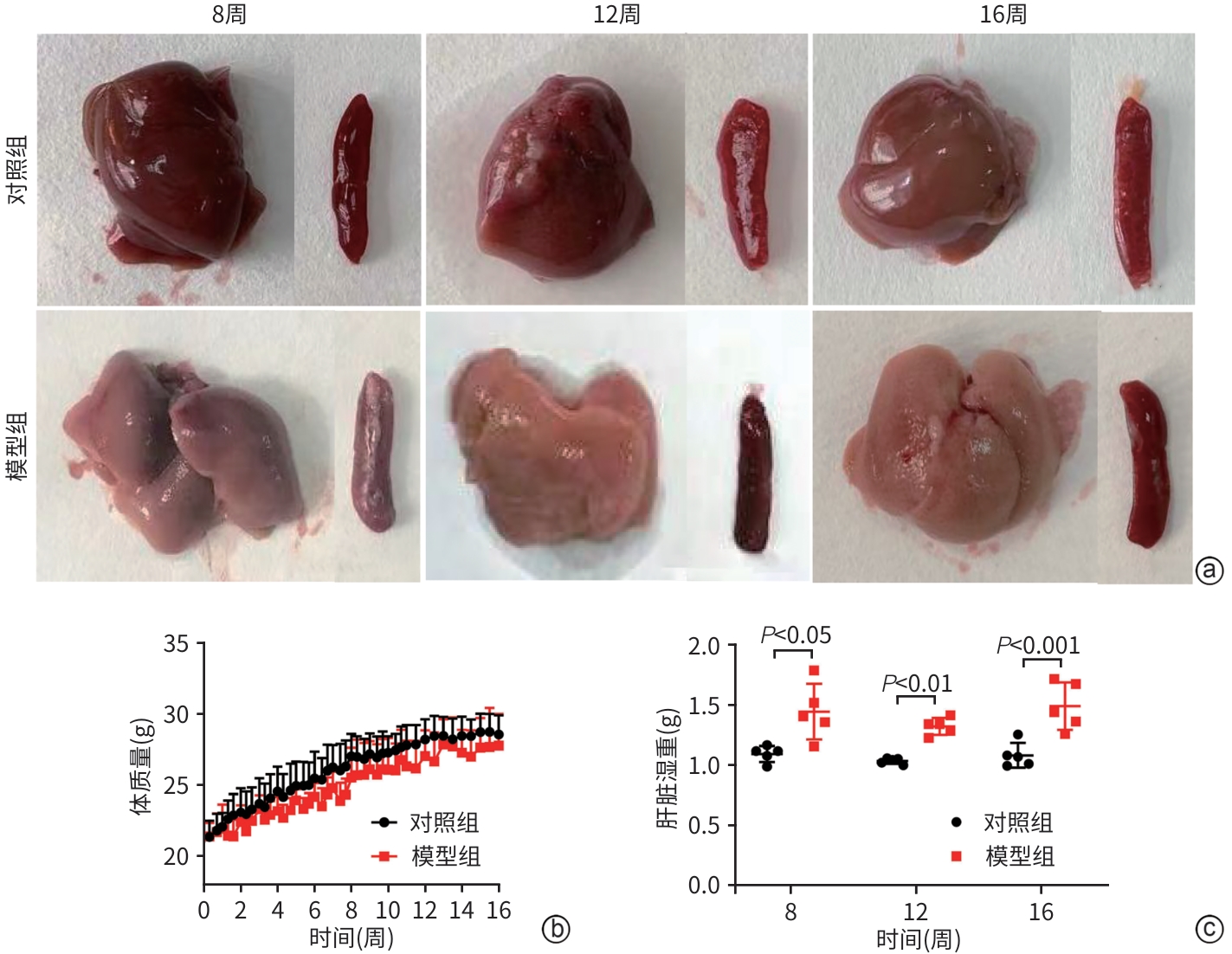
目的 比较高脂高糖高胆固醇联合腹腔注射10%四氯化碳(CCl4)诱导的代谢相关脂肪性肝炎(MASH)小鼠模型的肝脏胆汁酸谱与临床MASH胆汁酸谱的相似性,探究该模型用于药物干预MASH胆汁酸谱的可行性。 方法 30只C57BL/6J雄性小鼠随机分为对照组和模型组,每组各15只。对照组:正常饲料饮食,正常饮水,每周注射橄榄油;模型组:高脂高糖高胆固醇饮食,高糖溶液饮水,每周注射CCl4+橄榄油。第8、12、16周末从每组中各取5只小鼠处理、取材。评估小鼠行为学、称取体质量、肝湿重;通过肝组织HE染色、SAF评分、油红O染色及染色面积半定量分析、血清ALT与AST水平及肝脏TG检测,评价肝脏病变和脂质沉积;通过肝组织天狼星红染色,评价肝脏纤维化;采用超高效液相色谱-串联质谱靶向代谢组学法检测肝脏胆汁酸谱,包括胆酸(CA)、甘氨胆酸(GCA)、鹅去氧胆酸(CDCA)、甘氨鹅去氧胆酸(GCDCA)、熊去氧胆酸(UDCA)、牛磺熊去氧胆酸(TUDCA)、猪去氧胆酸(HDCA)和甘氨去氧胆酸(GDCA)。符合正态分布的计量资料两组间比较采用成组t检验;不符合正态分布计量资料组间比较使用Wilcox秩和检验。 结果 与同期对照组比较,第8、12、16周模型组小鼠毛发杂乱、无光泽,活动减弱,反应较迟缓;肝脏湿重均显著增加(P值均<0.05);血清ALT水平及肝脏TG水平显著升高(P值均<0.05),SAF评分显著升高(P值均<0.05)。肝组织中差异胆汁酸:第8周时,模型组中CA、CDCA显著高于对照组,UDCA、TUDCA、HDCA及GDCA低于对照组(P值均<0.05);第12周时,模型组CA、GCA、CDCA、GCDCA均显著高于对照组,UDCA、HDCA低于对照组(P值均<0.05);第16周时,模型组CA、GCA、CDCA、GCDCA、TUDCA均显著高于对照组,UDCA、HDCA、GDCA均低于对照组(P值均<0.05)。肝组织胆汁酸池中差异胆汁酸占比:第8周时,肝组织胆汁酸池差异胆汁酸中模型组CA、CDCA显著高于对照组,UDCA、TUDCA、GDCA、HDCA显著低于对照组(P值均<0.05);第12、16周时,模型组GCA、GCDCA显著高于对照组,UDCA、GDCA、HDCA显著低于对照组(P值均<0.05)。 结论 高脂高糖高胆固醇饮食联合CCl4诱导的MASH小鼠模型肝脏胆汁酸谱发生显著变化,与临床MASH胆汁酸变化具有较好的相似性,提示该模型可用于探究药物对MASH胆汁酸谱的干预作用。 -
关键词:
- 代谢相关脂肪性肝炎 /
- 胆汁酸类 /
- 小鼠, 近交C57BL
Abstract:Objective To compare the hepatic bile acid profile between a mouse model of metabolic-associated steatohepatitis (MASH) induced by a high-fat, high-sugar, and high-cholesterol diet combined with intraperitoneal injection of 10% carbon tetrachloride (CCl4) and MASH cases in clinical practice, and to investigate the feasibility of this model in studying drug interventions on bile acid profile in MASH. Methods A total of 30 male C57BL/6J mice were randomly divided into control group and model group, with 15 mice in each group. The mice in the control group were given normal diet and drinking water and weekly injections of olive oil, and those in the model group were given a high-fat, high-sugar, and high-cholesterol diet, high-sugar drinking water, and weekly injections of CCl4+olive oil. At the end of weeks 8, 12, and 16, 5 mice were selected from each group to collect samples. Behavioral assessments were performed, and body weight and liver wet weight were measured; liver pathology and lipid deposition were evaluated by HE staining, SAF scoring, oil Red O staining, the semi-quantitative analysis of stained area, the serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and liver triglyceride (TG) content; Sirius Red staining was performed for liver tissue to assess liver fibrosis; ultra-performance liquid chromatography-tandem mass spectrometry and targeted metabolomics were used to measure the hepatic bile acid profile, including cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), glycochenodeoxycholic acid (GCDCA), ursodeoxycholic acid (UDCA), tauroursodeoxycholic acid (TUDCA), hyodeoxycholic acid (HDCA), and glycodeoxycholic acid (GDCA). The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and the Wilcoxon rank-sum test was used for comparison of non-normally distributed continuous data between two groups. Results Compared with the control group at the same time point, the model group had disheveled and dull fur, reduced activity, and relatively slow reactions at weeks 8, 12, and 16, as well as significant increases in liver wet weight (P<0.05), the serum level of ALT (P<0.05), the content of TG in the liver (P<0.05), and SAF score (P<0.05). As for the differentially expressed bile acids in liver tissue, compared with the control group at week 8, the model group had significantly higher levels of CA and CDCA and significantly lower levels of UDCA, TUDCA, HDCA, and GDCA (all P<0.05); compared with the control group at week 12, the model group had significantly higher levels of CA, GCA, CDCA, and GCDCA and significantly lower levels of UDCA and HDCA (all P<0.05); compared with the control group at week 16, the model group had significantly higher levels of CA, GCA, CDCA, GCDCA, and TUDCA and significantly lower levels of UDCA, HDCA, and GDCA (all P<0.05). As for the differentially expressed bile acids in the bile acid pool of liver tissue, compared with the control group at week 8, the model group had significantly higher levels of CA and CDCA and significantly lower levels of UDCA, TUDCA, GDCA, and HDCA (all P<0.05); compared with the control group at weeks 12 and 16, the model group had significantly higher levels of GCA and GCDCA and significantly lower levels of UDCA, GDCA, and HDCA (all P<0.05). Conclusion There are significant changes in the hepatic bile acid profile in a mouse model of MASH induced by a high-fat, high-sugar, and high-cholesterol diet combined with CCl4, which are similar to the changes in bile acids in MASH cases in clinical practice, suggesting that this model can be used to explore the interventional effect of drugs on the bile acid profile in MASH. -
表 1 各组小鼠病理SAF评分
Table 1. Pathological SAF scores of mice in different groups
组别 动物数(只) 脂肪变性(分) 炎症(分) 气球样变(分) 纤维化(分) SAF评分 分值(分) Z值 P值 8周 -2.825 0.005 对照组 5 0 0 0 0 0 模型组 5 3(3~3) 2(2~2) 2(2~2) 2(2~2) 9(8~9) 12周 -2.835 0.005 对照组 5 0 0 0 0 0 模型组 5 3(2~3) 2(2~2) 2(2~2) 4(3~4) 9(8~9) 16周 -2.835 0.005 对照组 5 0 0 0 0 0 模型组 5 3(2~3) 2(2~2) 2(2~2) 4(3~4) 10(9~10) -
[1] TARGHER G, BYRNE CD, TILG H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications[J]. Gut, 2024, 73( 4): 691- 702. DOI: 10.1136/gutjnl-2023-330595. [2] YOUNOSSI Z, TACKE F, ARRESE M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis[J]. Hepatology, 2019, 69( 6): 2672- 2682. DOI: 10.1002/hep.30251. [3] PETTA S, TARGHER G, ROMEO S, et al. The first MASH drug therapy on the horizon: Current perspectives of resmetirom[J]. Liver Int, 2024, 44( 7): 1526- 1536. DOI: 10.1111/liv.15930. [4] RINELLA ME, NEUSCHWANDER-TETRI BA, SIDDIQUI MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease[J]. Hepatology, 2023, 77( 5): 1797- 1835. DOI: 10.1097/HEP.0000000000000323. [5] SHI L, JIN L, HUANG W. Bile acids, intestinal barrier dysfunction, and related diseases[J]. Cells, 2023, 12( 14): 1888. DOI: 10.3390/cells12141888. [6] WANG JJ, CAI XB, LU LG. Association between bile acids and nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 5): 1166- 1171. DOI: 10.3969/j.issn.1001-5256.2023.05.026.王俊俊, 蔡晓波, 陆伦根. 胆汁酸与非酒精性脂肪性肝病的关系[J]. 临床肝胆病杂志, 2023, 39( 5): 1166- 1171. DOI: 10.3969/j.issn.1001-5256.2023.05.026. [7] CHÁVEZ-TALAVERA O, HAAS J, GRZYCH G, et al. Bile acid alterations in nonalcoholic fatty liver disease, obesity, insulin resistance and type 2 diabetes: What do the human studies tell?[J]. Curr Opin Lipidol, 2019, 30( 3): 244- 254. DOI: 10.1097/MOL.0000000000000597. [8] POUPON R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: An overview of their mechanisms of action[J]. Clin Res Hepatol Gastroenterol, 2012, 36( Suppl 1): S3- S12. DOI: 10.1016/S2210-7401(12)70015-3. [9] ALKHOURI N, LACERTE C, EDWARDS J, et al. Safety, pharmacokinetics and pharmacodynamics of obeticholic acid in subjects with fibrosis or cirrhosis from NASH[J]. Liver Int, 2024, 44( 4): 966- 978. DOI: 10.1111/liv.15816. [10] DENK H, ABUJA PM, ZATLOUKAL K. Animal models of NAFLD from the pathologist’s point of view[J]. Biochim Biophys Acta BBA Mol Basis Dis, 2019, 1865( 5): 929- 942. DOI: 10.1016/j.bbadis.2018.04.024. [11] TSUCHIDA T, LEE YA, FUJIWARA N, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer[J]. J Hepatol, 2018, 69( 2): 385- 395. DOI: 10.1016/j.jhep.2018.03.011. [12] TSOUKA S, KUMAR P, SEUBNOOCH P, et al. Transcriptomics-driven metabolic pathway analysis reveals similar alterations in lipid metabolism in mouse MASH model and human[J]. Commun Med(Lond), 2024, 4( 1): 39. DOI: 10.1038/s43856-024-00465-3. [13] ZHANG LZ, SHI JW, SHEN Q, et al. Astragalus saponins protect against extrahepatic and intrahepatic cholestatic liver fibrosis models by activation of farnesoid X receptor[J]. J Ethnopharmacol, 2024, 318( Pt A): 116833. DOI: 10.1016/j.jep.2023.116833. [14] ZHANG LZ, JIANG XY, SHI JW, et al. Isoastragaloside I attenuates cholestatic liver diseases by ameliorating liver injury, regulating bile acid metabolism and restoring intestinal barrier[J]. J Ethnopharmacol, 2024, 335: 118649. DOI: 10.1016/j.jep.2024.118649. [15] European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease[J]. J Hepatol, 2016, 64( 6): 1388- 1402. DOI: 10.1016/j.jhep.2015.11.004. [16] Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of metabolic dysfunction-associated(non-alcoholic)fatty liver disease(Version 2024)[J]. J Pract Hepatol, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163.中华医学会肝病学分会. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 实用肝脏病杂志, 2024, 27( 4): 494- 510. DOI: 10.3760/cma.j.cn501113-20240327-00163. [17] ANGULO P, KLEINER DE, DAM-LARSEN S, et al. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2015, 149( 2): 389- 397. e 10. DOI: 10.1053/j.gastro.2015.04.043. [18] KLEINER DE, MAKHLOUF HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children[J]. Clin Liver Dis, 2016, 20( 2): 293- 312. DOI: 10.1016/j.cld.2015.10.011. [19] BERGER D, DESAI V, JANARDHAN S. Con: Liver biopsy remains the gold standard to evaluate fibrosis in patients with nonalcoholic fatty liver disease[J]. Clin Liver Dis(Hoboken), 2019, 13( 4): 114- 116. DOI: 10.1002/cld.740. [20] YANG HL, ZHANG DQ, CHEN GF, et al. Changes of serum and liver bile acid profiles in a mouse model of metabolic associated fatty liver disease induced by a methionine-choline-deficient diet[J]. Chin J Tissue Eng Res, 2021, 25( 26): 4137- 4144.杨海琳, 张定棋, 陈高峰, 等. 蛋氨酸-胆碱缺乏饮食诱导代谢相关脂肪肝模型小鼠血清和肝脏胆汁酸谱的变化[J]. 中国组织工程研究, 2021, 25( 26): 4137- 4144. [21] BRUNT EM, KLEINER DE, WILSON LA, et al. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: Results from the nonalcoholic steatohepatitis clinical research network treatment trials[J]. Hepatology, 2019, 70( 2): 522- 531. DOI: 10.1002/hep.30418. [22] KANG JH, LI MJ, LUAN PP, et al. Establishment of non-alcoholic steatohepatitis mouse model induced by Western diet combined with low-dose carbon tetrachloride[J]. J Shanghai Jiao Tong Univ Med Sci, 2020, 40( 5): 590- 597. DOI: 10.3969/j.issn.1674-8115.2020.05.005.康建华, 李明杰, 栾培培, 等. 西方饮食联合小剂量四氯化碳构建非酒精性脂肪性肝炎小鼠模型研究[J]. 上海交通大学学报(医学版), 2020, 40( 5): 590- 597. DOI: 10.3969/j.issn.1674-8115.2020.05.005. [23] BASUNI AA, SWEED D, ELGAZZAR MF, et al. Exploring serum bile acids as potential noninvasive biomarkers for nonalcoholic fatty liver disease[J]. Egypt Liver J, 2024, 14( 1): 70. DOI: 10.1186/s43066-024-00378-9. [24] FENG S, XIE XM, LI JC, et al. Bile acids induce liver fibrosis through the NLRP3 inflammasome pathway and the mechanism of FXR inhibition of NLRP3 activation[J]. Hepatol Int, 2024, 18( 3): 1040- 1052. DOI: 10.1007/s12072-023-10610-0. [25] LIN XZ, MAI MQ, HE TP, et al. Efficiency of ursodeoxycholic acid for the treatment of nonalcoholic steatohepatitis: A systematic review and meta-analysis[J]. Expert Rev Gastroenterol Hepatol, 2022, 16( 6): 537- 545. DOI: 10.1080/17474124.2022.2083605. [26] KUANG JL, WANG JY, LI YT, et al. Hyodeoxycholic acid alleviates non-alcoholic fatty liver disease through modulating the gut-liver axis[J]. Cell Metab, 2023, 35( 10): 1752- 1766. e 8. DOI: 10.1016/j.cmet.2023.07.011. [27] NIE QX, LUO X, WANG K, et al. Gut symbionts alleviate MASH through a secondary bile acid biosynthetic pathway[J]. Cell, 2024, 187( 11): 2717- 2734. e 33. DOI: 10.1016/j.cell.2024.03.034. [28] ZHENG LT, WANG Z, CHEN YC, et al. Role of Akkermansia muciniphila in nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2024, 40( 3): 594- 599. DOI: 10.12449/JCH240326.郑立婷, 汪哲, 陈玉春, 等. 嗜黏蛋白阿克曼菌在非酒精性脂肪性肝病中的作用[J]. 临床肝胆病杂志, 2024, 40( 3): 594- 599. DOI: 10.12449/JCH240326. -



 PDF下载 ( 4013 KB)
PDF下载 ( 4013 KB)

 下载:
下载: