mRNA疫苗:胰腺癌个性化治疗新曙光
DOI: 10.12449/JCH250404
-
摘要: 胰腺癌作为恶性程度最高的实体肿瘤之一,其5年生存率长期维持在约13%,且80%的患者在确诊时已失去手术机会。此外,传统放化疗及靶向治疗因肿瘤异质性高、免疫抑制微环境复杂而效果有限。近年来,mRNA疫苗凭借其独特的技术优势,成为肿瘤免疫治疗的新焦点。基于非整合性mRNA模板,mRNA疫苗可精准编码肿瘤新抗原,在宿主体内高效表达并诱导多维度免疫应答。针对胰腺癌,研究热点集中在肿瘤相关抗原疫苗及肿瘤特异性抗原疫苗的研发与优化。当前研究主要关注基于测序的新抗原表位优化、靶向递送技术和人工智能驱动的免疫应答预测模型,以期推动mRNA疫苗在胰腺癌精准治疗中的应用。未来研究需进一步突破肿瘤微环境中关键免疫抑制分子的靶向阻断,精准识别肿瘤特异性抗原表位,开发高效新型疫苗,为胰腺癌患者带来新的治疗希望。Abstract: Pancreatic cancer is currently recognized as one of the most malignant solid tumors, with a 5-year survival rate of 13% over a long period of time, and 80% of the patients have lost the opportunity for surgery at the time of confirmed diagnosis. In addition, the efficacy of conventional radiochemotherapy and targeted therapy is limited by high tumor heterogeneity and the complex immunosuppressive microenvironment. In recent years, mRNA vaccines have become a new focus of tumor immunotherapy due to their unique technical advantages. Based on non-integrating mRNA templates, mRNA vaccines enable precise encoding of tumor neoantigens, which are efficiently expressed in the host and can induce multifaceted immune responses. As for pancreatic cancer, current studies mainly focus on the development and optimization of tumor-associated antigen vaccines and tumor-specific antigen vaccines, as well as next-generation sequencing-guided neoantigen epitope optimization, innovative targeted delivery systems, and artificial intelligence-powered predictive models for immune response, thereby promoting the application of mRNA vaccines in the precise treatment of pancreatic cancer. Further studies should make breakthroughs in the targeted blockade of critical immunosuppressive molecules within the tumor microenvironment, the precise identification of tumor-specific antigenic epitopes, and the development of highly efficient vaccines, so as to bring new hopes for patients with pancreatic cancer.
-
Key words:
- Pancreatic Neoplasms /
- mRNA Vaccine /
- Immunotherapy, Active
-
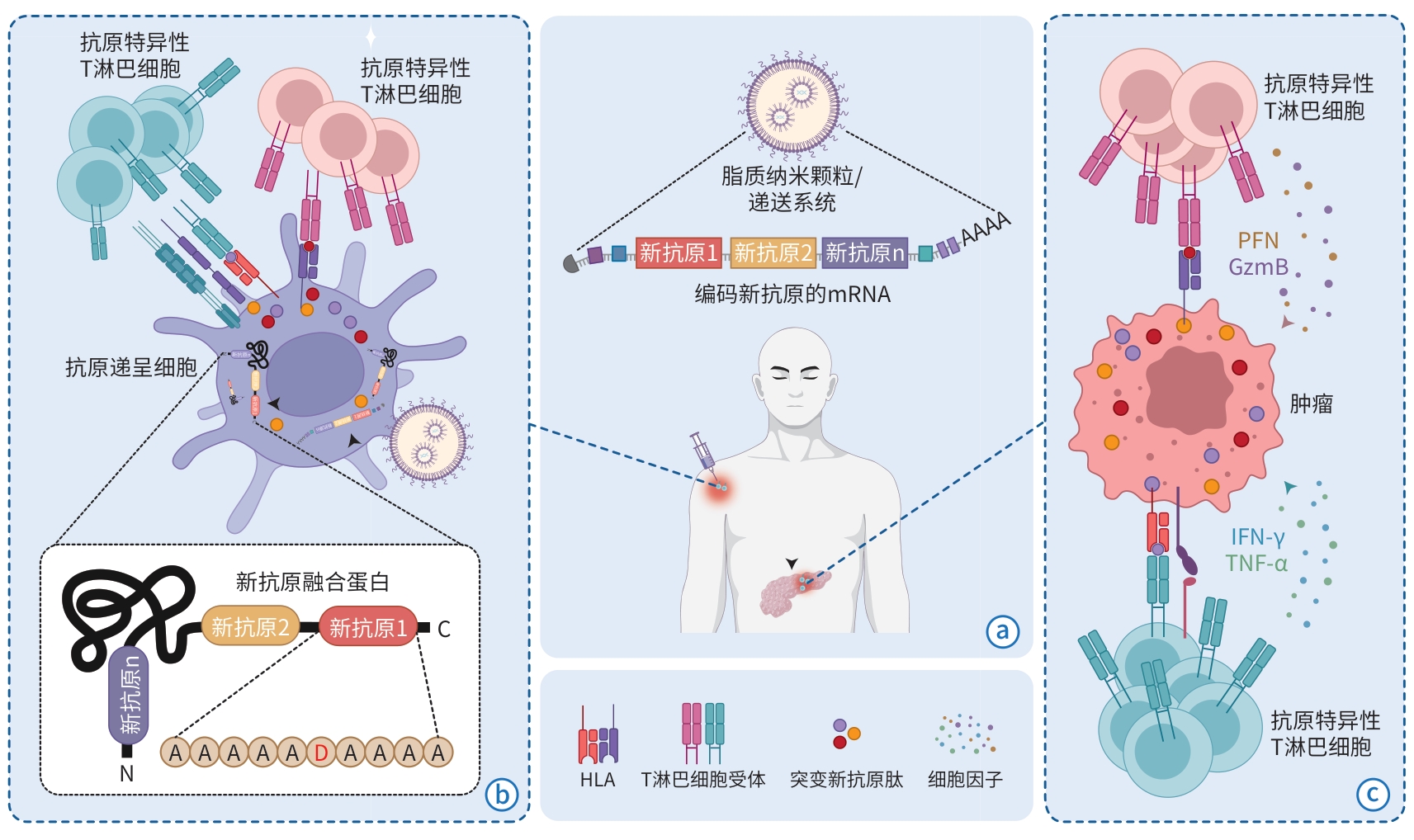
注: a,mRNA疫苗:串联多个个性化肿瘤新抗原序列的mRNA被脂质纳米颗粒或其他递送系统包裹后成药。b,抗原递呈细胞端的新抗原翻译、加工和T淋巴细胞激活:mRNA被抗原递呈细胞内吞后,经过内逃逸进入细胞质,被翻译成包含多个肿瘤新抗原氨基酸片段的融合蛋白。融合蛋白经蛋白酶体切割后,成为突变新抗原肽,被匹配的人类白细胞抗原(HLA)递呈到细胞表面,随后引起抗原特异性T淋巴细胞的激活和扩增。c,肿瘤细胞端的T淋巴细胞激活、扩增和杀伤:激活的T淋巴细胞迁移至肿瘤组织中,识别肿瘤细胞表面被呈递的突变抗原-HLA复合物,进一步激活T淋巴细胞下游通路后不断扩增的同时,活化并释放大量细胞因子,起到杀伤肿瘤细胞的效果。
图 1 肿瘤mRNA疫苗的作用机制
Figure 1. Mechanism map of tumor mRNA vaccine
表 1 胰腺癌mRNA疫苗的临床试验汇总
Table 1. Summary of clinical trials of pancreatic cancer mRNA vaccine
疫苗名称 所属公司 类别 机制 适应证 阶段 试验号 BNT122 BioNTech/Genentech 个性化 20种患者特异性抗原,联用PD-L1 胰腺癌术后 Ⅱ期 NCT05968326 BNT122 BioNTech/Genentech 个性化 20种患者特异性抗原,联用PD-1 包括胰腺癌在内的晚期实体肿瘤 Ⅰ期 NCT03289962 mRNA-0217/S001 上海交通大学附属瑞金医院 个性化 1~20种个性化肿瘤新抗原 包括胰腺癌在内的晚期实体肿瘤 IIT NCT05916248 LK101 立康生命 个性化 mRNA疫苗+树突状细胞载体 包括胰腺癌在内的晚期实体肿瘤 Ⅰ期 NCT06054932 XP-004 上海交通大学附属瑞金医院 个性化 1~20种个性化肿瘤新抗原 胰腺癌术后 IIT NCT06496373 PANC-IIT-RGL 复旦大学附属肿瘤医院 个性化 个性化肿瘤新抗原 胰腺癌术后 IIT NCT06156267 mRNA-5671 Moderna 通用型 多个KRAS突变抗原串联 晚期非小细胞肺癌、结肠、胰腺癌 Ⅰ期 NCT03948763 mRNA-4359 Moderna 通用型 靶向免疫检查点IDO/PD-L1 包括胰腺癌在内的晚期实体肿瘤 Ⅰ/Ⅱ期 NCT05533697 GRT-C903/GRT-R904 Gritstone 通用型 16~20个固定抗原/单独靶向KRAS,联用PD-1/CTLA-4 包括胰腺癌在内的晚期实体肿瘤 Ⅰ/Ⅱ期 NCT03953235 ABO2102 上海交通大学附属瑞金医院 通用型 多个KRAS突变抗原串联 晚期胰腺癌 IIT NCT06577532 注:PD-L1,程序性死亡配体;PD-1,程序性死亡受体;IDO,吲哚胺2,3-双加氧酶;CTLA-4,细胞毒性T淋巴细胞相关抗原4;IIT,研究者发起的临床试验。
-
[1] SIEGEL RL, GIAQUINTO AN, JEMAL A. Cancer statistics, 2024[J]. CA Cancer J Clin, 2024, 74( 1): 12- 49. DOI: 10.3322/caac.21820. [2] RYAN DP, HONG TS, BARDEESY N. Pancreatic adenocarcinoma[J]. N Engl J Med, 2014, 371( 11): 1039- 1049. DOI: 10.1056/nejmra1404198. [3] SIEGEL RL, MILLER KD, WAGLE NS, et al. Cancer statistics, 2023[J]. CA A Cancer J Clinicians, 2023, 73( 1): 17- 48. DOI: 10.3322/caac.21763. [4] QIN Q, YU R, ERIKSSON JE, et al. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma therapy: Challenges and opportunities[J]. Cancer Lett, 2024, 591: 216859. DOI: 10.1016/j.canlet.2024.216859. [5] GROSSBERG AJ, CHU LC, DEIG CR, et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma[J]. CA Cancer J Clin, 2020, 70( 5): 375- 403. DOI: 10.3322/caac.21626. [6] TORPHY RJ, FUJIWARA Y, SCHULICK RD. Pancreatic cancer treatment: Better, but a long way to go[J]. Surg Today, 2020, 50( 10): 1117- 1125. DOI: 10.1007/s00595-020-02028-0. [7] STOOP TF, THEIJSE RT, SEELEN LWF, et al. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer[J]. Nat Rev Gastroenterol Hepatol, 2024, 21( 2): 101- 124. DOI: 10.1038/s41575-023-00856-2. [8] SPRINGFELD C, JÄGER D, BÜCHLER MW, et al. Chemotherapy for pancreatic cancer[J]. Presse Med, 2019, 48( 3 Pt 2): e159- e174. DOI: 10.1016/j.lpm.2019.02.025. [9] MIZRAHI JD, SURANA R, VALLE JW, et al. Pancreatic cancer[J]. Lancet, 2020, 395( 10242): 2008- 2020. DOI: 10.1016/s0140-6736(20)30974-0. [10] WOOD LD, CANTO MI, JAFFEE EM, et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment[J]. Gastroenterology, 2022, 163( 2): 386- 402. e 1. DOI: 10.1053/j.gastro.2022.03.056. [11] LIU XX, LI Z, WANG YX. Advances in targeted therapy and immunotherapy for pancreatic cancer[J]. Adv Biol(Weinh), 2021, 5( 3): e1900236. DOI: 10.1002/adbi.201900236. [12] KOLBEINSSON HM, CHANDANA S, WRIGHT GP, et al. Pancreatic cancer: A review of current treatment and novel therapies[J]. J Invest Surg, 2023, 36( 1): 2129884. DOI: 10.1080/08941939.2022.2129884. [13] HU ZI, O’REILLY EM. Therapeutic developments in pancreatic cancer[J]. Nat Rev Gastroenterol Hepatol, 2024, 21( 1): 7- 24. DOI: 10.1038/s41575-023-00840-w. [14] YE ZF, HARMON J, NI W, et al. The mRNA vaccine revolution: COVID-19 has launched the future of vaccinology[J]. ACS Nano, 2023, 17( 16): 15231- 15253. DOI: 10.1021/acsnano.2c12584. [15] YADAV T, KUMAR S, MISHRA G, et al. Tracking the COVID-19 vaccines: The global landscape[J]. Hum Vaccin Immunother, 2023, 19( 1): 2191577. DOI: 10.1080/21645515.2023.2191577. [16] LI MC, WANG H, TIAN LL, et al. COVID-19 vaccine development: Milestones, lessons and prospects[J]. Signal Transduct Target Ther, 2022, 7( 1): 146. DOI: 10.1038/s41392-022-00996-y. [17] LAMB YN. BNT162b2 mRNA COVID-19 vaccine: First approval[J]. Drugs, 2021, 81( 4): 495- 501. DOI: 10.1007/s40265-021-01480-7. [18] WANG X, HAEUSSLER K, SPELLMAN A, et al. Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines in immunocompromised individuals: A systematic review and meta-analysis using the GRADE framework[J]. Front Immunol, 2023, 14: 1204831. DOI: 10.3389/fimmu.2023.1204831. [19] TEO SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273[J]. J Pharm Pract, 2022, 35( 6): 947- 951. DOI: 10.1177/08971900211009650. [20] GRAÑA C, GHOSN L, EVRENOGLOU T, et al. Efficacy and safety of COVID-19 vaccines[J]. Cochrane Database Syst Rev, 2022, 12( 12): CD015477. DOI: 10.1002/14651858.CD015477. [21] CHANG SL, LIU HB, WU J, et al. Effectiveness of BNT162b2 and mRNA-1273 vaccines against COVID-19 infection: A meta-analysis of test-negative design studies[J]. Vaccines(Basel), 2022, 10( 3): 469. DOI: 10.3390/vaccines10030469. [22] YUAN Y, GAO F, CHANG Y, et al. Advances of mRNA vaccine in tumor: A maze of opportunities and challenges[J]. Biomark Res, 2023, 11( 1): 6. DOI: 10.1186/s40364-023-00449-w. [23] WANG Y, ZHANG ZQ, LUO JW, et al. mRNA vaccine: A potential therapeutic strategy[J]. Mol Cancer, 2021, 20( 1): 33. DOI: 10.1186/s12943-021-01311-z. [24] LI YH, WANG MN, PENG XQ, et al. mRNA vaccine in cancer therapy: Current advance and future outlook[J]. Clin Transl Med, 2023, 13( 8): e1384. DOI: 10.1002/ctm2.1384. [25] BERGMAN H, BUCKLEY BS, VILLANUEVA G, et al. Comparison of different human papillomavirus(HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males[J]. Cochrane Database Syst Rev, 2019, 2019( 11): CD013479. DOI: 10.1002/14651858.CD013479. [26] LEE S, YOON H, HONG SH, et al. mRNA-HPV vaccine encoding E6 and E7 improves therapeutic potential for HPV-mediated cancers via subcutaneous immunization[J]. J Med Virol, 2023, 95( 12): e29309. DOI: 10.1002/jmv.29309. [27] SAHIN U, OEHM P, DERHOVANESSIAN E, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma[J]. Nature, 2020, 585( 7823): 107- 112. DOI: 10.1038/s41586-020-2537-9. [28] ALKHATIB O, MILES T, JONES RP, et al. Current and future genomic applications for surgeons[J]. Ann R Coll Surg Engl, 2024, 106( 4): 321- 328. DOI: 10.1308/rcsann.2024.0031. [29] LORENTZEN CL, HAANEN JB, MET Ö, et al. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment[J]. Lancet Oncol, 2022, 23( 10): e450- e458. DOI: 10.1016/S1470-2045(22)00372-2. [30] LIU C, SHI QQ, HUANG XG, et al. mRNA-based cancer therapeutics[J]. Nat Rev Cancer, 2023, 23( 8): 526- 543. DOI: 10.1038/s41568-023-00586-2. [31] WOLFF JA, MALONE RW, WILLIAMS P, et al. Direct gene transfer into mouse muscle in vivo[J]. Science, 1990, 247( 4949 Pt 1): 1465- 1468. DOI: 10.1126/science.1690918. [32] KARIKÓ K, BUCKSTEIN M, NI HP, et al. Suppression of RNA recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA[J]. Immunity, 2005, 23( 2): 165- 175. DOI: 10.1016/j.immuni.2005.06.008. [33] GAUTAM SK, KUMAR S, DAM V, et al. MUCIN-4(MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy[J]. Semin Immunol, 2020, 47: 101391. DOI: 10.1016/j.smim.2020.101391. [34] CAI ZY, WURI Q, SONG Y, et al. CircRNA-loaded DC vaccine in combination with low-dose gemcitabine induced potent anti-tumor immunity in pancreatic cancer model[J]. Cancer Immunol Immunother, 2025, 74( 2): 68. DOI: 10.1007/s00262-024-03924-x. [35] GENG F, DONG L, BAO X, et al. CAFs/tumor cells co-targeting DNA vaccine in combination with low-dose gemcitabine for the treatment of Panc02 murine pancreatic cancer[J]. Mol Ther Oncolytics, 2022, 26: 304- 313. DOI: 10.1016/j.omto.2022.07.008. [36] BUONAGURO L, TAGLIAMONTE M. Selecting target antigens for cancer vaccine development[J]. Vaccines(Basel), 2020, 8( 4): 615. DOI: 10.3390/vaccines8040615. [37] BITOUNIS D, JACQUINET E, ROGERS MA, et al. Strategies to reduce the risks of mRNA drug and vaccine toxicity[J]. Nat Rev Drug Discov, 2024, 23( 4): 281- 300. DOI: 10.1038/s41573-023-00859-3. [38] FU Q, ZHAO XM, HU JX, et al. mRNA vaccines in the context of cancer treatment: From concept to application[J]. J Transl Med, 2025, 23( 1): 12. DOI: 10.1186/s12967-024-06033-6. [39] WEI J, HUI AM. The paradigm shift in treatment from Covid-19 to oncology with mRNA vaccines[J]. Cancer Treat Rev, 2022, 107: 102405. DOI: 10.1016/j.ctrv.2022.102405. [40] WANG XJ, WANG W, ZOU SY, et al. Combination therapy of KRAS G12V mRNA vaccine and pembrolizumab: Clinical benefit in patients with advanced solid tumors[J]. Cell Res, 2024, 34( 9): 661- 664. DOI: 10.1038/s41422-024-00990-9. [41] ROJAS LA, SETHNA Z, SOARES KC, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer[J]. Nature, 2023, 618( 7963): 144- 150. DOI: 10.1038/s41586-023-06063-y. [42] MIAO L, ZHANG Y, HUANG L. mRNA vaccine for cancer immunotherapy[J]. Mol Cancer, 2021, 20( 1): 41. DOI: 10.1186/s12943-021-01335-5. [43] HUANG X, ZHANG G, TANG TY, et al. Personalized pancreatic cancer therapy: From the perspective of mRNA vaccine[J]. Mil Med Res, 2022, 9( 1): 53. DOI: 10.1186/s40779-022-00416-w. [44] CARVALHO T. Personalized anti-cancer vaccine combining mRNA and immunotherapy tested in melanoma trial[J]. Nat Med, 2023, 29( 10): 2379- 2380. DOI: 10.1038/d41591-023-00072-0. [45] BIRD L. mRNA vaccine for treating pancreatic cancer[J]. Nat Rev Immunol, 2023, 23( 7): 413. DOI: 10.1038/s41577-023-00899-1. -



 PDF下载 ( 1097 KB)
PDF下载 ( 1097 KB)

 下载:
下载:


