肝衰竭肠道屏障损伤与环鸟苷酸-腺苷酸合酶(cGAS)-干扰素基因刺激因子(STING)信号通路的关系
DOI: 10.12449/JCH250328
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:宫嫚负责课题设计,拟定写作思路,指导撰写文章并最后定稿;张宁、周超参与收集整理文献材料,修改论文;唐巧负责撰写论文。
Association between intestinal barrier disruption in liver failure and the cGAS-STING signaling pathway
-
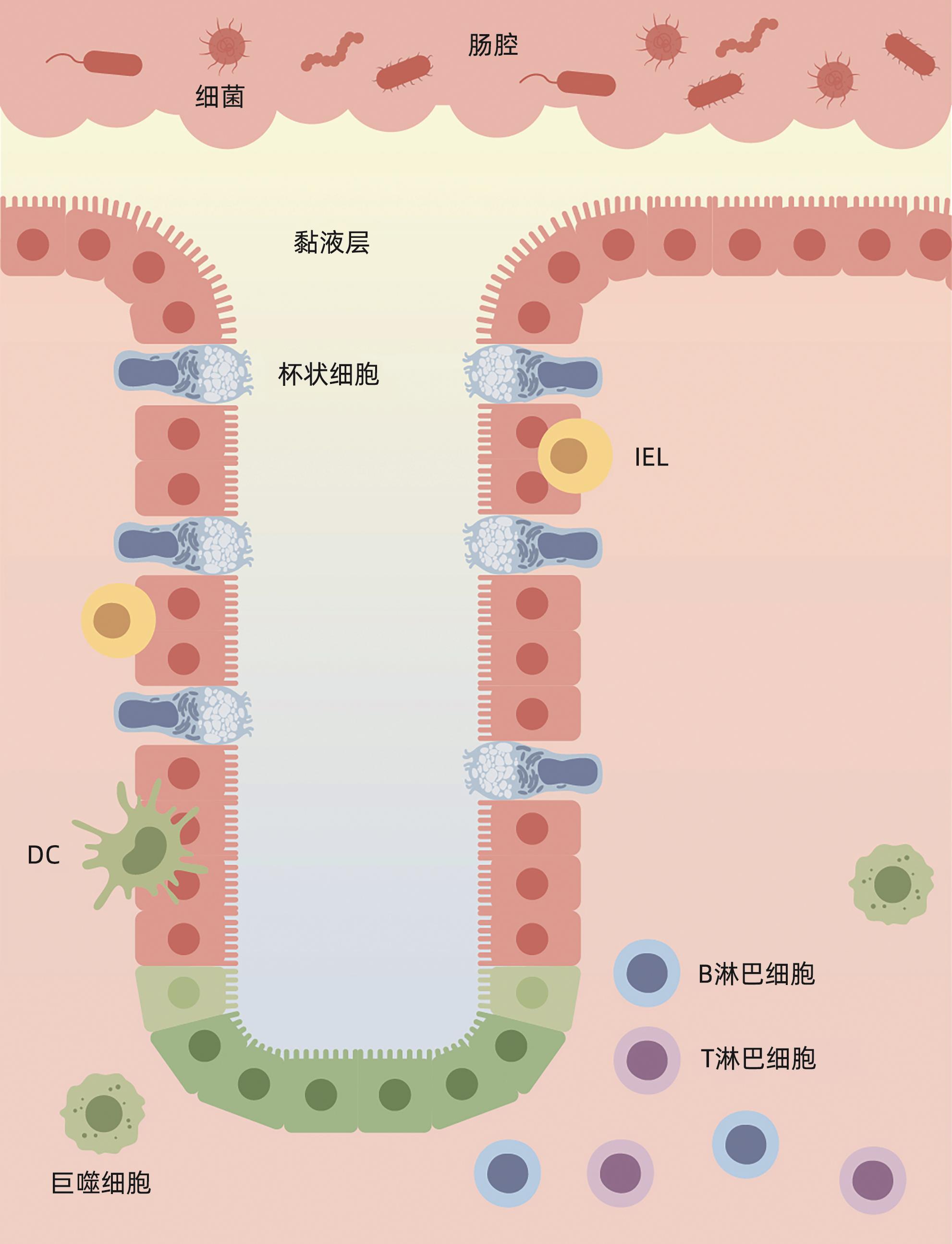
摘要: 肝衰竭是常见的严重肝病综合征,病死率高。肠道屏障作为一个整体,其功能和结构的完整性与肝衰竭发生发展密切相关。环鸟苷酸-腺苷酸合酶(cGAS)-干扰素基因刺激因子(STING)信号通路通过识别病原体入侵及宿主自身细胞损伤产生的DNA,诱导Ⅰ型干扰素的产生,参与先天免疫反应。大量研究表明,cGAS-STING通路的激活对肠道屏障的细胞结构、黏膜成分及共生菌群均能产生影响。本文概述了cGAS-STING信号通路与肝衰竭肠道屏障损伤之间的关系,希望为临床肝衰竭的治疗提供新思路。Abstract: Liver failure is a common severe syndrome of liver diseases with high mortality. The function and structural integrity of the intestinal barrier as an entity are closely associated with the development and progression of liver failure. The cGAS-STING signaling pathway can participate in innate immune response by recognizing DNA produced by pathogen invasion and host cell damage and inducing the production of type I interferon. Numerous studies have shown that activation of the cGAS-STING pathway can significantly impact the cellular structure, mucosal components, and commensal bacteria of the intestinal barrier. This article reviews the interplay between the cGAS-STING signaling pathway and intestinal barrier disruption in liver failure, in order to provide novel insights for the clinical management of liver failure.
-
Key words:
- Liver Failure /
- Gastrointestinal Microbiome /
- Signaling Pathway
-
[1] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association of Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [2] CHEN MJ, LI X, TANG SH. Research progress in multi-dimensional evaluation of liver function in patients with liver failure[J]. Clin J Med Offic, 2023, 51( 9): 901- 903, 907. DOI: 10.16680/j.1671-3826.2023.09.05.陈美娟, 李雪, 汤善宏. 多维度评估肝功能在肝衰竭患者预后中研究进展[J]. 临床军医杂志, 2023, 51( 9): 901- 903, 907. DOI: 10.16680/j.1671-3826.2023.09.05. [3] CHOPYK DM, GRAKOUI A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders[J]. Gastroenterology, 2020, 159( 3): 849- 863. DOI: 10.1053/j.gastro.2020.04.077. [4] QIANG R, LIU XZ, XU JC. The immune pathogenesis of acute-on-chronic liver failure and the danger hypothesis[J]. Front Immunol, 2022, 13: 935160. DOI: 10.3389/fimmu.2022.935160. [5] GAN Y, LI XY, HAN SZ, et al. The cGAS/STING pathway: A novel target for cancer therapy[J]. Front Immunol, 2022, 12: 795401. DOI: 10.3389/fimmu.2021.795401. [6] ALLAIRE JM, CROWLEY SM, LAW HT, et al. The intestinal epithelium: Central coordinator of mucosal immunity[J]. Trends Immunol, 2018, 39( 9): 677- 696. DOI: 10.1016/j.it.2018.04.002. [7] DI TOMMASO N, GASBARRINI A, PONZIANI FR. Intestinal barrier in human health and disease[J]. Int J Environ Res Public Health, 2021, 18( 23): 12836. DOI: 10.3390/ijerph182312836. [8] PAONE P, CANI PD. Mucus barrier, mucins and gut microbiota: The expected slimy partners?[J]. Gut, 2020, 69( 12): 2232- 2243. DOI: 10.1136/gutjnl-2020-322260. [9] HENDRIKX T, SCHNABL B. Antimicrobial proteins: Intestinal guards to protect against liver disease[J]. J Gastroenterol, 2019, 54( 3): 209- 217. DOI: 10.1007/s00535-018-1521-8. [10] LITVAK Y, MON KKZ, NGUYEN H, et al. Commensal enterobacteriaceae protect against Salmonella colonization through oxygen competition[J]. Cell Host Microbe, 2019, 25( 1): 128- 139. e 5. DOI: 10.1016/j.chom.2018.12.003. [11] HIIPPALA K, JOUHTEN H, RONKAINEN A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation[J]. Nutrients, 2018, 10( 8): 988. DOI: 10.3390/nu10080988. [12] VANCAMELBEKE M, VERMEIRE S. The intestinal barrier: A fundamental role in health and disease[J]. Expert Rev Gastroenterol Hepatol, 2017, 11( 9): 821- 834. DOI: 10.1080/17474124.2017.1343143. [13] WEI Q, HUANG H. Insights into the role of cell-cell junctions in physiology and disease[J]. Int Rev Cell Mol Biol, 2013, 306: 187- 221. DOI: 10.1016/B978-0-12-407694-5.00005-5. [14] MOWAT AM, AGACE WW. Regional specialization within the intestinal immune system[J]. Nat Rev Immunol, 2014, 14( 10): 667- 685. DOI: 10.1038/nri3738. [15] DELFINI M, STAKENBORG N, VIOLA MF, et al. Macrophages in the gut: Masters in multitasking[J]. Immunity, 2022, 55( 9): 1530- 1548. DOI: 10.1016/j.immuni.2022.08.005. [16] MARTÍNEZ-LÓPEZ M, IBORRA S, CONDE-GARROSA R, et al. Microbiota sensing by mincle-syk axis in dendritic cells regulates interleukin-17 and-22 production and promotes intestinal barrier integrity[J]. Immunity, 2019, 50( 2): 446- 461. e 9. DOI: 10.1016/j.immuni.2018.12.020. [17] TEZUKA H, OHTEKI T. Regulation of IgA production by intestinal dendritic cells and related cells[J]. Front Immunol, 2019, 10: 1891. DOI: 10.3389/fimmu.2019.01891. [18] SPENCER J, BEMARK M. Human intestinal B cells in inflammatory diseases[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 4): 254- 265. DOI: 10.1038/s41575-023-00755-6. [19] MANN ER, LAM YK, UHLIG HH. Short-chain fatty acids: Linking diet, the microbiome and immunity[J]. Nat Rev Immunol, 2024, 24( 8): 577- 595. DOI: 10.1038/s41577-024-01014-8. [20] LE N, MAZAHERY C, NGUYEN K, et al. Regulation of intestinal epithelial barrier and immune function by activated T cells[J]. Cell Mol Gastroenterol Hepatol, 2021, 11( 1): 55- 76. DOI: 10.1016/j.jcmgh.2020.07.004. [21] YOO JS, OH SF. Unconventional immune cells in the gut mucosal barrier: Regulation by symbiotic microbiota[J]. Exp Mol Med, 2023, 55( 9): 1905- 1912. DOI: 10.1038/s12276-023-01088-9. [22] GIL-CRUZ C, PEREZ-SHIBAYAMA C, ONDER L, et al. Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs[J]. Nat Immunol, 2016, 17( 12): 1388- 1396. DOI: 10.1038/ni.3566. [23] HOU QH, HUANG JX, AYANSOLA H, et al. Intestinal stem cells and immune cell relationships: Potential therapeutic targets for inflammatory bowel diseases[J]. Front Immunol, 2021, 11: 623691. DOI: 10.3389/fimmu.2020.623691. [24] SONNENBERG GF, MONTICELLI LA, ALENGHAT T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria[J]. Science, 2012, 336( 6086): 1321- 1325. DOI: 10.1126/science.1222551. [25] LE BOURHIS L, MARTIN E, PÉGUILLET I, et al. Antimicrobial activity of mucosal-associated invariant T cells[J]. Nat Immunol, 2010, 11( 8): 701- 708. DOI: 10.1038/ni.1890. [26] OLIVARES-VILLAGÓMEZ D, VAN KAER L. Intestinal intraepithelial lymphocytes: Sentinels of the mucosal barrier[J]. Trends Immunol, 2018, 39( 4): 264- 275. DOI: 10.1016/j.it.2017.11.003. [27] KAYAMA H, OKUMURA R, TAKEDA K. Interaction between the microbiota, epithelia, and immune cells in the intestine[J]. Annu Rev Immunol, 2020, 38: 23- 48. DOI: 10.1146/annurev-immunol-070119-115104. [28] CHEN BR, NI X, SUN R, et al. Commensal bacteria-dependent CD8αβ+ T cells in the intestinal epithelium produce antimicrobial peptides[J]. Front Immunol, 2018, 9: 1065. DOI: 10.3389/fimmu.2018.01065. [29] HOYTEMA VAN KONIJNENBURG DP, REIS BS, PEDICORD VA, et al. Intestinal epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection[J]. Cell, 2017, 171( 4): 783- 794. e 13. DOI: 10.1016/j.cell.2017.08.046. [30] WELLS JM, BRUMMER RJ, DERRIEN M, et al. Homeostasis of the gut barrier and potential biomarkers[J]. Am J Physiol Gastrointest Liver Physiol, 2017, 312( 3): G171- G193. DOI: 10.1152/ajpgi.00048.2015. [31] ZHANG B, DILIHUMAER ZYE, ZHANG SY, et al. Progress on pathogenesis and medical treatment of hepatitis B virus-related chronic and acute liver failure[J/CD]. Chin J Liver Dis(Electronic Version), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005.张斌, 迪丽胡玛尔·扎依尔, 张诗雨, 等. 乙型肝炎相关慢加急性肝衰竭发病机制及治疗进展[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 1): 28- 33. DOI: 10.3969/j.issn.1674-7380.2023.01.005. [32] BIGGINS SW, ANGELI P, GARCIA-TSAO G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases[J]. Hepatology, 2021, 74( 2): 1014- 1048. DOI: 10.1002/hep.31884. [33] KIM SE, PARK JW, KIM HS, et al. The role of gut dysbiosis in acute-on-chronic liver failure[J]. Int J Mol Sci, 2021, 22( 21): 11680. DOI: 10.3390/ijms222111680. [34] BAJAJ JS, VARGAS HE, REDDY KR, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis[J]. Clin Gastroenterol Hepatol, 2019, 17( 4): 756- 765. e 3. DOI: 10.1016/j.cgh.2018.07.022. [35] FERNÁNDEZ J, ACEVEDO J, WIEST R, et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis[J]. Gut, 2018, 67( 10): 1870- 1880. DOI: 10.1136/gutjnl-2017-314240. [36] PHILIPS CA, AUGUSTINE P. Gut barrier and microbiota in cirrhosis[J]. J Clin Exp Hepatol, 2022, 12( 2): 625- 638. DOI: 10.1016/j.jceh.2021.08.027. [37] FENG X, LIU DY, LI ZY, et al. Bioactive modulators targeting STING adaptor in cGAS-STING pathway[J]. Drug Discov Today, 2020, 25( 1): 230- 237. DOI: 10.1016/j.drudis.2019.11.007. [38] BAI JL, LIU F. The cGAS-cGAMP-STING pathway: A molecular link between immunity and metabolism[J]. Diabetes, 2019, 68( 6): 1099- 1108. DOI: 10.2337/dbi18-0052. [39] CHEN RH, DU JM, ZHU H, et al. The role of cGAS-STING signalling in liver diseases[J]. JHEP Rep, 2021, 3( 5): 100324. DOI: 10.1016/j.jhepr.2021.100324. [40] LUTHER J, KHAN S, GALA MK, et al. Hepatic gap junctions amplify alcohol liver injury by propagating cGAS-mediated IRF3 activation[J]. Proc Natl Acad Sci USA, 2020, 117( 21): 11667- 11673. DOI: 10.1073/pnas.1911870117. [41] ZHANG H. The role of autophagy and macrophage polarization mediated by STING pathway activation in the pathogenesis of HBV-related acute liver failure and the establishment of clinical prognosis model[D]. Hefei: Anhui Medical University, 2023.张浩. STING通路活化介导自噬及巨噬细胞极化在HBV相关慢加急性肝衰竭发病机制的作用及临床预后模型的建立[D]. 合肥: 安徽医科大学, 2023. [42] YU T, CHENG HR, LI XL, et al. Design and synthesis of hederagenin derivatives modulating STING/NF-κB signaling for the relief of acute liver injury in septic mice[J]. Eur J Med Chem, 2023, 245( Pt 1): 114911. DOI: 10.1016/j.ejmech.2022.114911. [43] CANESSO MCC, LEMOS L, NEVES TC, et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation[J]. Mucosal Immunol, 2018, 11( 3): 820- 834. DOI: 10.1038/mi.2017.88. [44] LOUIE A, BHANDULA V, PORTNOY DA. Secretion of c-di-AMP by Listeria monocytogenes leads to a STING-dependent antibacterial response during enterocolitis[J]. Infect Immun, 2020, 88( 12): e00407-20. DOI: 10.1128/IAI.00407-20. [45] ZHANG Q, CHEN QY, YAN CS, et al. The absence of STING ameliorates non-alcoholic fatty liver disease and reforms gut bacterial community[J]. Front Immunol, 2022, 13: 931176. DOI: 10.3389/fimmu.2022.931176. [46] ZHANG XF, WU J, LIU QJ, et al. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion[J]. Cell Death Dis, 2020, 11( 12): 1050. DOI: 10.1038/s41419-020-03239-6. [47] AL-SADI R, GUO SH, YE DM, et al. TNF-α modulation of intestinal tight junction permeability is mediated by NIK/IKK-α axis activation of the canonical NF-κB pathway[J]. Am J Pathol, 2016, 186( 5): 1151- 1165. DOI: 10.1016/j.ajpath.2015.12.016. [48] HU QY, REN HJ, LI GW, et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis[J]. EBioMedicine, 2019, 41: 497- 508. DOI: 10.1016/j.ebiom.2019.02.055. [49] MARTIN GR, BLOMQUIST CM, HENARE KL, et al. Stimulator of interferon genes(STING) activation exacerbates experimental colitis in mice[J]. Sci Rep, 2019, 9( 1): 14281. DOI: 10.1038/s41598-019-50656-5. [50] SCHAUPP L, MUTH S, ROGELL L, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells[J]. Cell, 2020, 181( 5): 1080- 1096. e 19. DOI: 10.1016/j.cell.2020.04.022. [51] GUTIERREZ-MERINO J, ISLA B, COMBES T, et al. Beneficial bacteria activate type-I interferon production via the intracellular cytosolic sensors STING and MAVS[J]. Gut Microbes, 2020, 11( 4): 771- 788. DOI: 10.1080/19490976.2019.1707015. -



 PDF下载 ( 937 KB)
PDF下载 ( 937 KB)

 下载:
下载:


