没食子酸对人肝癌HepG2细胞增殖、迁移、侵袭和凋亡的影响及其机制
DOI: 10.12449/JCH250315
Effect and mechanism of gallic acid on the proliferation, migration, invasion, and apoptosis of human hepatocellular carcinoma HepG2 cells
-
摘要:
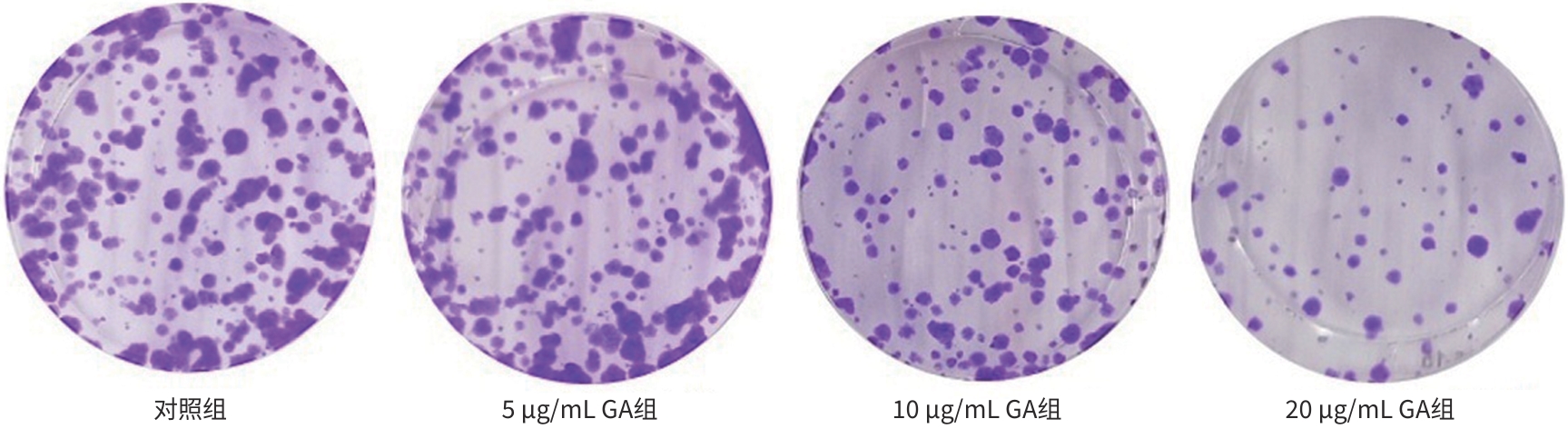
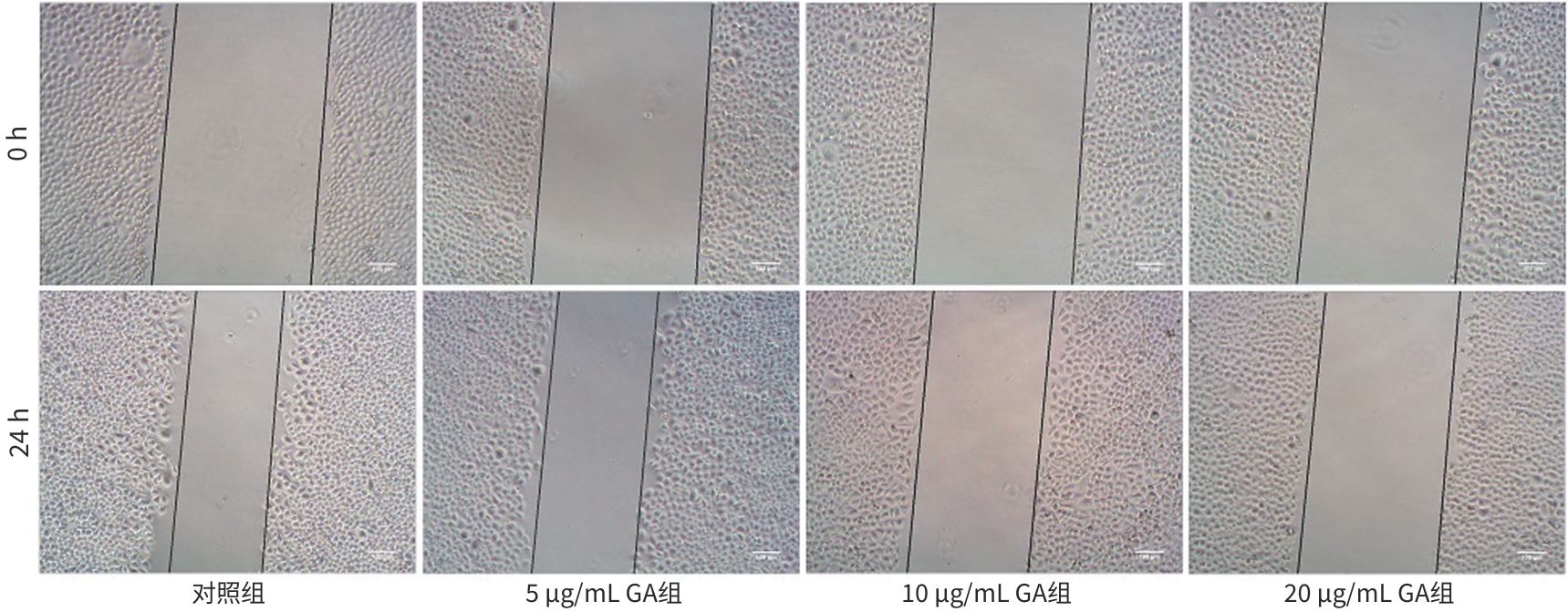
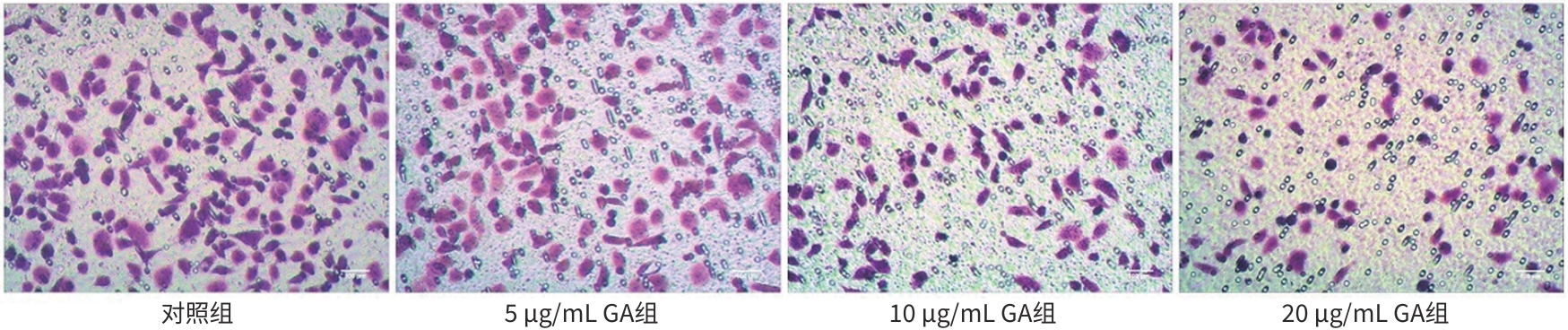
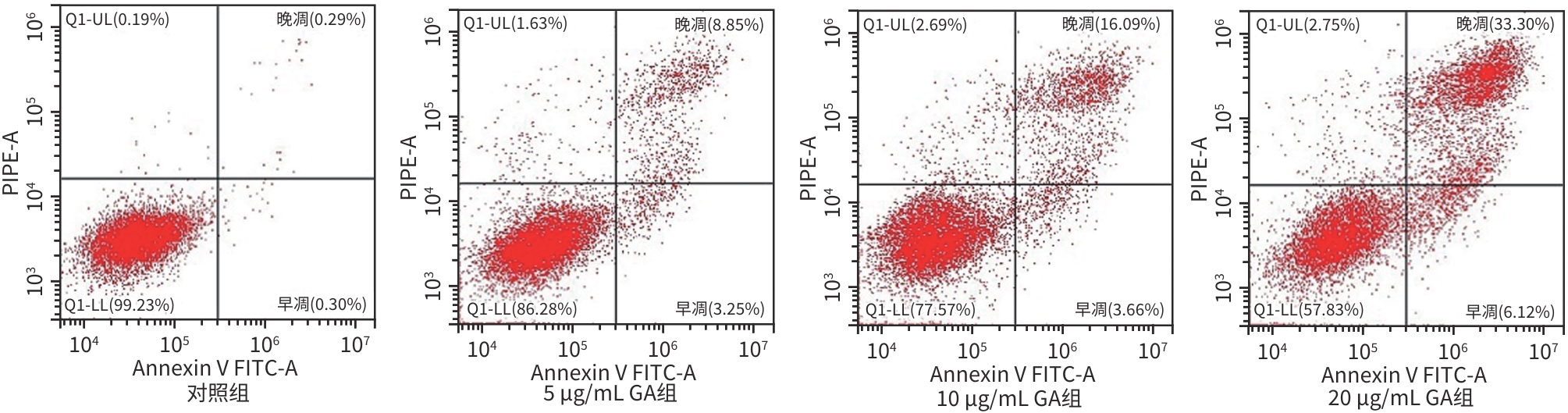
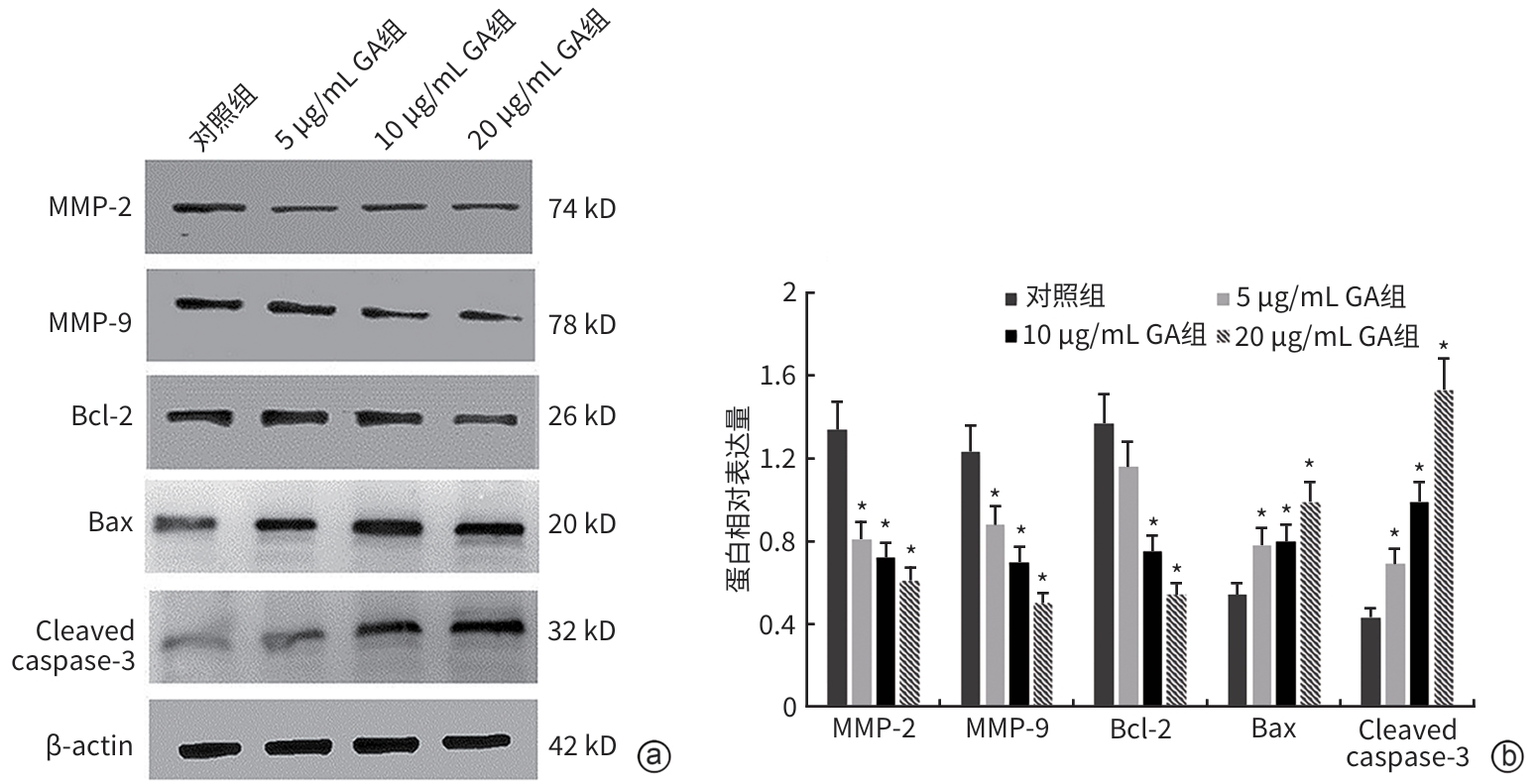
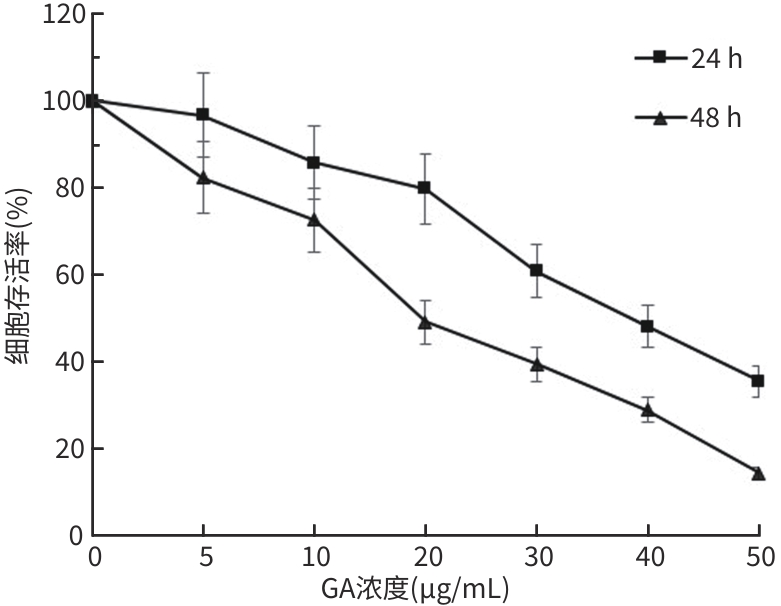
目的 观察没食子酸(GA)对人肝癌HepG2细胞增殖、迁移、侵袭和凋亡的影响,并探讨其作用机制。 方法 用不同浓度GA(0、5、10、20、30、40、50 μg/mL)处理肝癌HepG2细胞24 h和48 h后,CCK-8法检测细胞活性并计算IC50值;实验分为对照组(HepG2细胞)、5 μg/mL GA组、10 μg/mL GA组、20 μg/mL GA组,平板克隆形成实验检测GA对细胞增殖能力的影响,细胞划痕和Transwell小室侵袭实验检测GA对细胞迁移和侵袭能力的影响,流式细胞仪检测GA对细胞凋亡的影响;Western Blot检测基质金属蛋白酶2(MMP-2)、MMP-9和凋亡相关蛋白表达情况。多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 GA作用HepG2细胞24 h和48 h的IC50值为(38.02±2.58)μg/mL和(18.36±1.54)μg/mL。对照组、5 μg/mL GA组、10 μg/mL GA组、20 μg/mL GA组的细胞克隆形成数分别为(239.00±29.45)个、(210.00±19.00)个、(144.33±16.03)个、(57.00±9.55)个,与对照组比较,各实验组细胞克隆形成能力均明显下降(P值均<0.05)。处理24 h后,各组细胞的迁移率分别为42.62%±7.82%、35.34%±6.42%、21.85%±4.42%、12.57%±3.54%,穿膜细胞数目分别为(230.30±15.30)个、(182.12±12.60)个、(137.20±7.50)个、(124.40±6.80)个,与对照组比较,各实验组细胞的相对迁移率和穿膜细胞数均明显下降(P值均<0.05)。处理48 h后,各组细胞凋亡率分别为0.67%±0.08%、13.27%±1.07%、20.94%±2.45%、40.74%±2.63%,与对照组比较,各实验组细胞凋亡率均明显升高(P值均<0.05)。与对照组比较,各实验组细胞MMP-2、MMP-9表达水平均明显降低(P值均<0.05),Bcl-2关联X蛋白(Bax)、裂解的半胱氨酸天冬氨酸蛋白酶3(Cleaved caspase-3)蛋白表达水平均明显升高(P值均<0.05)。 结论 GA可抑制HepG2细胞增殖、迁移和侵袭,促进其凋亡,作用机制可能与调控Bax/Bcl-2以及迁移相关蛋白MMP-2、MMP-9有关。 Abstract:Objective To investigate the effect of gallic acid (GA) on the proliferation, migration, invasion, and apoptosis of human hepatocellular carcinoma HepG2 cells and its mechanism. Methods HepG2 cells were treated with different concentrations of GA (0, 5, 10, 20, 30, 40, and 50 μg/mL) for 24 and 48 hours, and CCK8 assay was used to measure cell viability and calculate IC50. The experiment was divided into control group (HepG2 cells), 5 μg/mL GA group, 10 μg/mL GA group, and 20 μg/mL GA group. Plate colony formation assay was used to evaluate the effect of GA on cell proliferation; wound healing assay and Transwell chamber assay were used to observe the effect of GA on cell migration and invasion; flow cytometry was used to observe the effect of GA on cell apoptosis; Western blot was used to measure the expression of matrix metallopeptidase-2 (MMP-2), matrix metallopeptidase-9 (MMP-9), and apoptosis-related proteins. A one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results The mean IC50 value of GA on HepG2 cells was 38.02±2.58 μg/mL at 24 hours and 18.36±1.54 μg/mL at 48 hours. The number of cell colonies was 239.00±29.45 in the control group, 210.00±19.00 in the 5 μg/mL GA group, 144.33±16.03 in the 10 μg/mL GA group, and 57.00±9.55 in the 20 μg/mL GA group, suggesting that compared with the control group, each GA group had a significant reduction in cell colony formation ability (all P<0.05). After 24 hours of treatment, the cell migration rate was 42.62%± 7.82% in the control group, 35.34%±6.42% in the 5 μg/mL GA group, 21.85%±4.42% in the 10 μg/mL GA group, and 12.57%± 3.54% in the 20 μg/mL GA group, respectively, in these four groups, and the number of transmembrane cells in these four groups was 230.30±15.30, 182.12±12.60, 137.20±7.50, and 124.40±6.80, respectively, suggesting that compared with the control group, each GA group had significant reductions in migration rate and the number of transmembrane cells (all P<0.05). After 48 hours of treatment, the cell apoptotic rate was 0.67%±0.08% in the control group, 13.27%±1.07% in the 5 μg/mL GA group, 20.94%± 2.45% in the 10 μg/mL GA group, and 40.74%±2.63% in the 20 μg/mL GA group, and compared with the control group, each GA group had a significant increase in cell apoptosis rate (all P<0.05). Compared with the control group, each GA group had significant reductions in the protein expression levels of MMP-2 and MMP-9 (all P<0.05) and significant increases in the protein expression levels of Bax and cleaved caspase-3 (all P<0.05). Conclusion GA can inhibit the proliferation, migration, and invasion of HepG2 cells and promote the apoptosis of HepG2 cells, possibly by regulating MMP-2, MMP-9, and the apoptosis-related proteins Bax/Bcl-2. -
Key words:
- Gallic Acid /
- Liver Neoplasms, Experimental /
- Cell Proliferation /
- Cell Movement /
- Apoptosis
-
表 1 各组HepG2细胞迁移率和穿膜细胞数
Table 1. Migration rate and number invasion of HepG2 cells in each group
组别 迁移率(η/%) 穿膜细胞数(个) 对照组 42.62±7.82 230.30±15.30 5 μg/mL GA组 35.34±6.421) 182.12±12.601) 10 μg/mL GA组 21.85±4.421) 137.20±7.501) 20 μg/mL GA组 12.57±3.541) 124.40±6.801) F值 40.030 82.926 P值 <0.001 <0.001 注:与对照组比较,1)P<0.05。
-
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] JIN AH, ZHU JB, YIN XZ, et al. Effect of iridoid glycosides from Boschniakia rossica on epithelial-mesenchymal transition of HepG2 cells induced by transforming growth factor-beta 1[J]. J Clin Hepatol, 2024, 40( 6): 1175- 1182. DOI: 10.12449/JCH240617.金爱花, 朱洁波, 尹学哲, 等. 草苁蓉环烯醚萜苷(IGBR)对TGF-β1诱导的HepG2细胞上皮间质转化模型的影响[J]. 临床肝胆病杂志, 2024, 40( 6): 1175- 1182. DOI: 10.12449/JCH240617. [3] XIE DY, SHI JY, ZHOU J, et al. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: A Chinese perspective[J]. Clin Mol Hepatol, 2023, 29( 2): 206- 216. DOI: 10.3350/cmh.2022.0402. [4] FAN R, PAPATHEODORIDIS G, SUN J, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis[J]. J Hepatol, 2020, 73( 6): 1368- 1378. DOI: 10.1016/j.jhep.2020.07.025. [5] ZHOU J, SUN HC, WANG Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma(2019 edition)[J]. Liver Cancer, 2020, 9( 6): 682- 720. DOI: 10.1159/000509424. [6] SUN GL, WANG D. Gallic acid from Terminalia chebula inhibited the growth of esophageal carcinoma cells by suppressing the Hippo signal pathway[J]. Iran J Basic Med Sci, 2020, 23( 11): 1401- 1408. DOI: 10.22038/ijbms.2020.42283.9982. [7] CHOI HJ, SONG JH, BHATT LR, et al. Anti-human rhinovirus activity of Gallic acid possessing antioxidant capacity[J]. Phytother Res, 2010, 24( 9): 1292- 1296. DOI: 10.1002/ptr.3101. [8] LIN XM, WANG GF, LIU P, et al. Gallic acid suppresses colon cancer proliferation by inhibiting SRC and EGFR phosphorylation[J]. Exp Ther Med, 2021, 21( 6): 638. DOI: 10.3892/etm.2021.10070. [9] ZHANG TX, MA LJ, WU PF, et al. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway[J]. Oncol Rep, 2019, 41( 3): 1779- 1788. DOI: 10.3892/or.2019.6976. [10] YANG XL, XUE JH, CHEN TY, et al. Effect of atractylone on the viability and apoptosis of hepatoma HepG2 cells and related mechanism[J]. J Clin Hepatol, 2021, 37( 11): 2589- 2594. DOI: 10.3969/j.issn.1001-5256.2021.11.020.杨雪丽, 薛建华, 陈天阳, 等. 苍术酮对肝癌HepG2细胞活性、凋亡的影响及其相关机制[J]. 临床肝胆病杂志, 2021, 37( 11): 2589- 2594. DOI: 10.3969/j.issn.1001-5256.2021.11.020. [11] QU Y, ZHANG WJ, CHEN FF, et al. Progress in research and development of chemical drugs and treatment of liver cancer[J]. Gansu Sci Technol, 2023, 39( 8): 114- 119, 127. DOI: 10.3969/j.issn.1000-0952.2023.08.028.屈延, 张文杰, 陈芳芳, 等. 肝癌的化学药物研发及治疗进展[J]. 甘肃科技, 2023, 39( 8): 114- 119, 127. DOI: 10.3969/j.issn.1000-0952.2023.08.028. [12] ZHAO YY, HAN ZQ, ZOU YP, et al. Effect of lysophosphatidic acid on hepatoma cells and related mechanism[J]. J Clin Hepatol, 2023, 39( 11): 2623- 2628. DOI: 10.3969/j.issn.1001-5256.2023.11.016.赵燕颖, 韩振琦, 邹艳平, 等. 溶血磷脂酸(LPA)对肝癌细胞的影响及相关机制的初步探讨[J]. 临床肝胆病杂志, 2023, 39( 11): 2623- 2628. DOI: 10.3969/j.issn.1001-5256.2023.11.016. [13] XU HC, WANG FL, XIE LH. Current status and perspectives in clinical treatment of intermediate and advanced primary hepatocellular carcinoma[J]. J Changchun Univ Chin Med, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024.许华晨, 王凤玲, 谢林虎. 中晚期原发性肝细胞癌的临床治疗现状与展望[J]. 长春中医药大学学报, 2024, 40( 1): 103- 107. DOI: 10.13463/j.cnki.cczyy.2024.01.024. [14] YANG C, ZHANG HL, ZHANG LM, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 4): 203- 222. DOI: 10.1038/s41575-022-00704-9. [15] WU H, NULAN BDL, LIU LY, et al. Inhibitory effects of Gallic acid on human esophageal cancer TE-1 cells in vitro and its mechanism[J]. China Pharm, 2022, 33( 12): 1448- 1454. DOI: 10.6039/j.issn.1001-0408.2022.12.07.吴昊, 努兰·拜都拉, 刘琳玉, 等. 没食子酸对人食管癌TE-1细胞的体外抑制作用及其机制[J]. 中国药房, 2022, 33( 12): 1448- 1454. DOI: 10.6039/j.issn.1001-0408.2022.12.07. [16] HAN QQ, YE MR, JIN QL. Demethylzeylasteral inhibits proliferation, migration and invasion and promotes apoptosis of non-small cell lung cancer cells by inhibiting the AKT/CREB signaling pathway[J]. J South Med Univ, 2024, 44( 2): 280- 288. DOI: 10.12122/j.issn.1673-4254.2024.02.10.韩齐齐, 叶梦然, 金齐力. 去甲泽拉木醛通过抑制AKT/CREB信号通路抑制非小细胞肺癌细胞的增殖、迁移和侵袭[J]. 南方医科大学学报, 2024, 44( 2): 280- 288. DOI: 10.12122/j.issn.1673-4254.2024.02.10. [17] XU GS, JIANG HB, PAN J, et al. Inhibitory effects of betulinic acid on migration and invasion of gastric cancer MGC-803 cells and their mechanisms[J]. J Jilin Univ(Med Edit), 2022, 48( 1): 122- 128. DOI: 10.13481/j.1671-587X.20220115.许广松, 蒋海兵, 盘箐, 等. 桦木酸对胃癌MGC-803细胞迁移和侵袭的抑制作用及其机制[J]. 吉林大学学报(医学版), 2022, 48( 1): 122- 128. DOI: 10.13481/j.1671-587X.20220115. [18] JIANG B, YANG T, FENG LF, et al. Effects of salidroside on proliferation, migration, invasion and apoptosis of 97H cells[J]. Chin Pharmacol Bull, 2023, 39( 3): 445- 452. DOI: 10.12360/CPB202202019.蒋兵, 杨韬, 封龙飞, 等. 红景天苷对97H细胞增殖、迁移、侵袭及凋亡的影响[J]. 中国药理学通报, 2023, 39( 3): 445- 452. DOI: 10.12360/CPB202202019. [19] PANG LL, HU Y, LUO J, et al. Study of the mechanism of combretastatin a-4 derivative LGD5 induced G2/M cycle arrest and apoptosis in human cervical cancer HeLa cells[J]. Chin J Clin Pharmacol Ther, 2024, 29( 10): 1100- 1109. DOI: 10.12092/j.issn.1009-2501.2024.10.003.庞丽丽, 胡莹, 罗洁, 等. CA-4类衍生物LGD5诱导人宫颈癌HeLa细胞发生G2/M周期阻滞和凋亡的机制研究[J]. 中国临床药理学与治疗学, 2024, 29( 10): 1100- 1109. DOI: 10.12092/j.issn.1009-2501.2024.10.003. [20] YUAN CL, CHEN GP, JING CB, et al. Eriocitrin, a dietary flavonoid suppressed cell proliferation, induced apoptosis through modulation of JAK2/STAT3 and JNK/p38 MAPKs signaling pathway in MCF-7 cells[J]. J Biochem Mol Toxicol, 2022, 36( 1): e22943. DOI: 10.1002/jbt.22943. [21] WANG ZY, ZHANG H, ZHOU JH, et al. Eriocitrin from lemon suppresses the proliferation of human hepatocellular carcinoma cells through inducing apoptosis and arresting cell cycle[J]. Cancer Chemother Pharmacol, 2016, 78( 6): 1143- 1150. DOI: 10.1007/s00280-016-3171-y. [22] ZHOU H, ZHANG YQ, GAN C, et al. Eriocitrin suppresses proliferation and migration of hepatocellular carcinoma SMMC-7721 cells by promoting ROS production and activating the MAPK pathway[J]. J South Med Univ, 2023, 43( 3): 412- 419. DOI: 10.12122/j.issn.1673-4254.2023.03.11.周慧, 张雨晴, 甘超, 等. 圣草次苷抑制肝细胞癌SMMC-7721细胞的增殖和迁移: 基于激活ROS/MAPKs信号轴[J]. 南方医科大学学报, 2023, 43( 3): 412- 419. DOI: 10.12122/j.issn.1673-4254.2023.03.11. -



 PDF下载 ( 3143 KB)
PDF下载 ( 3143 KB)

 下载:
下载: