Go-ichi-ni-san复合物亚基1(GINS1)对肝细胞癌进展和化疗耐药的影响
DOI: 10.12449/JCH250314
Effect of Go-Ichi-Ni-San complex subunit 1 on disease progression and chemotherapy resistance in hepatocellular carcinoma
-
摘要:
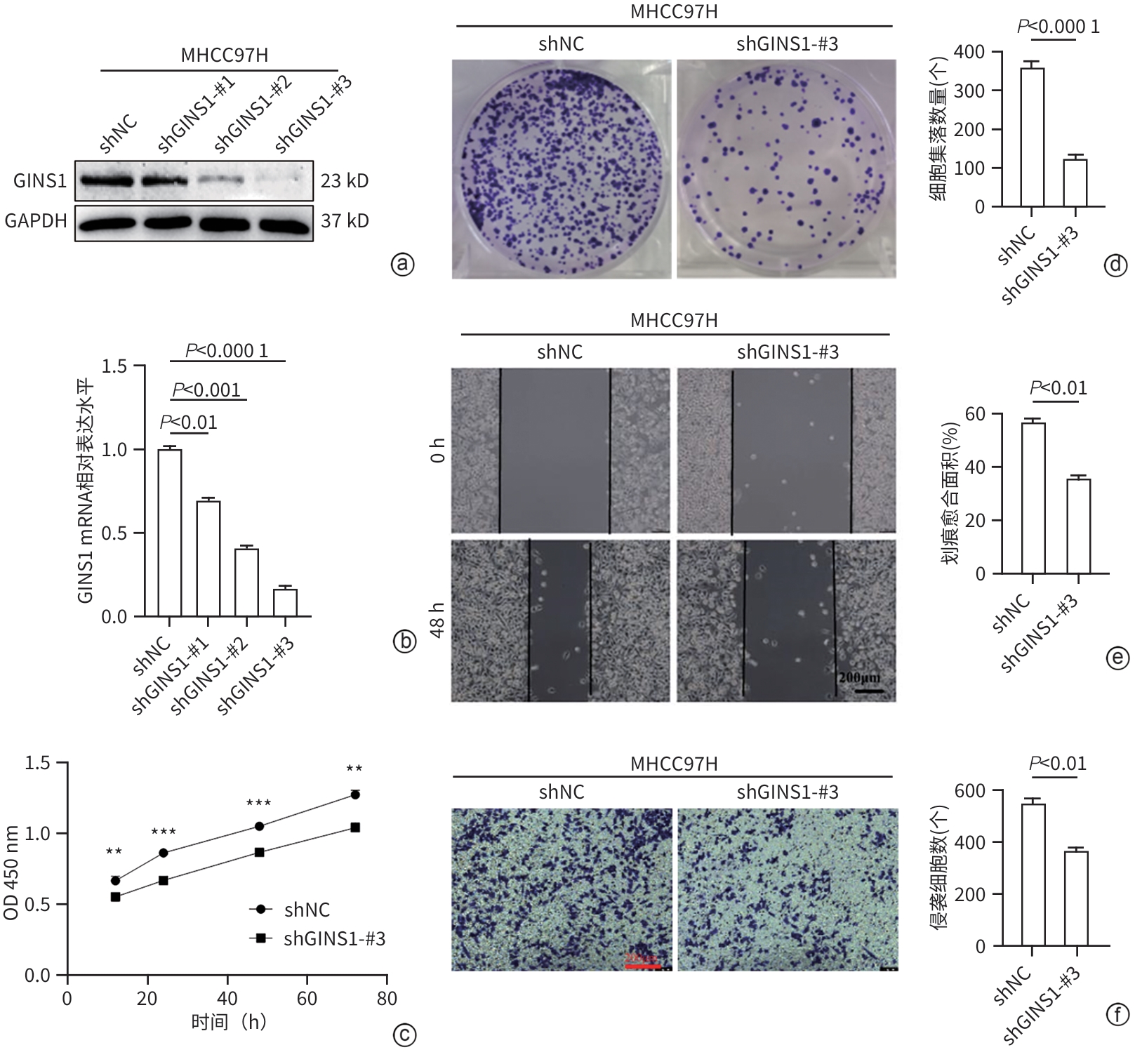
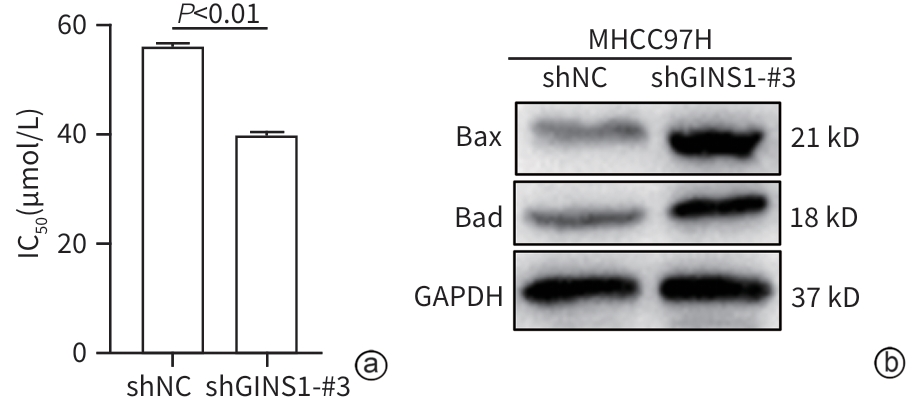
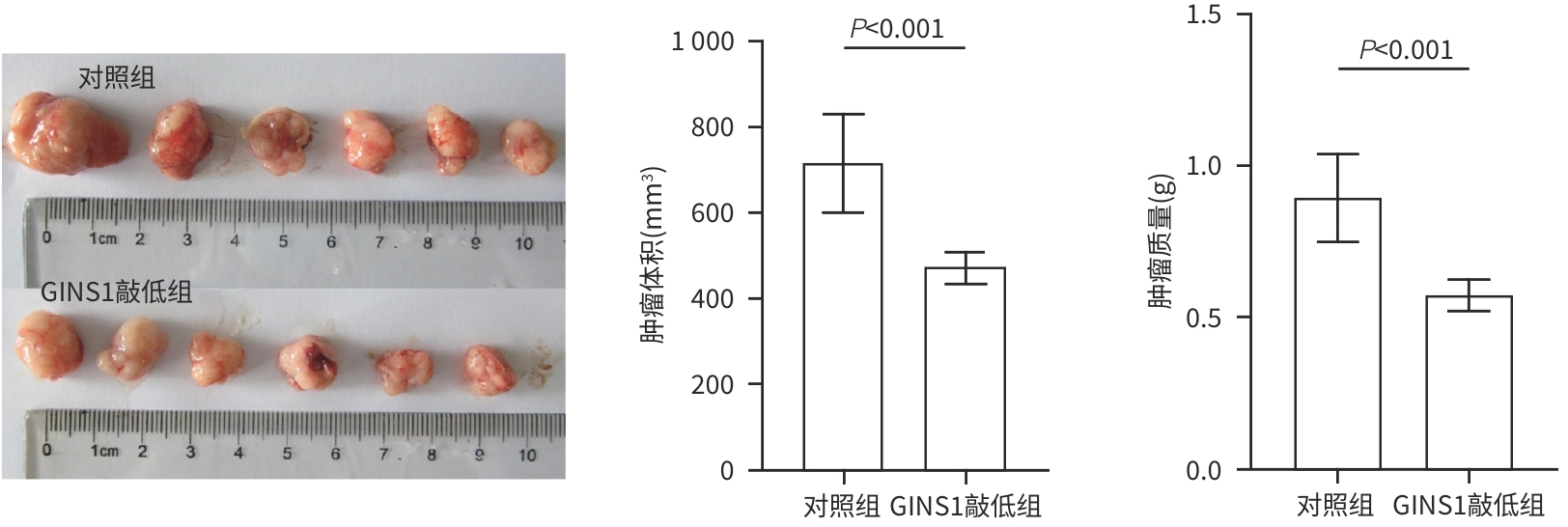
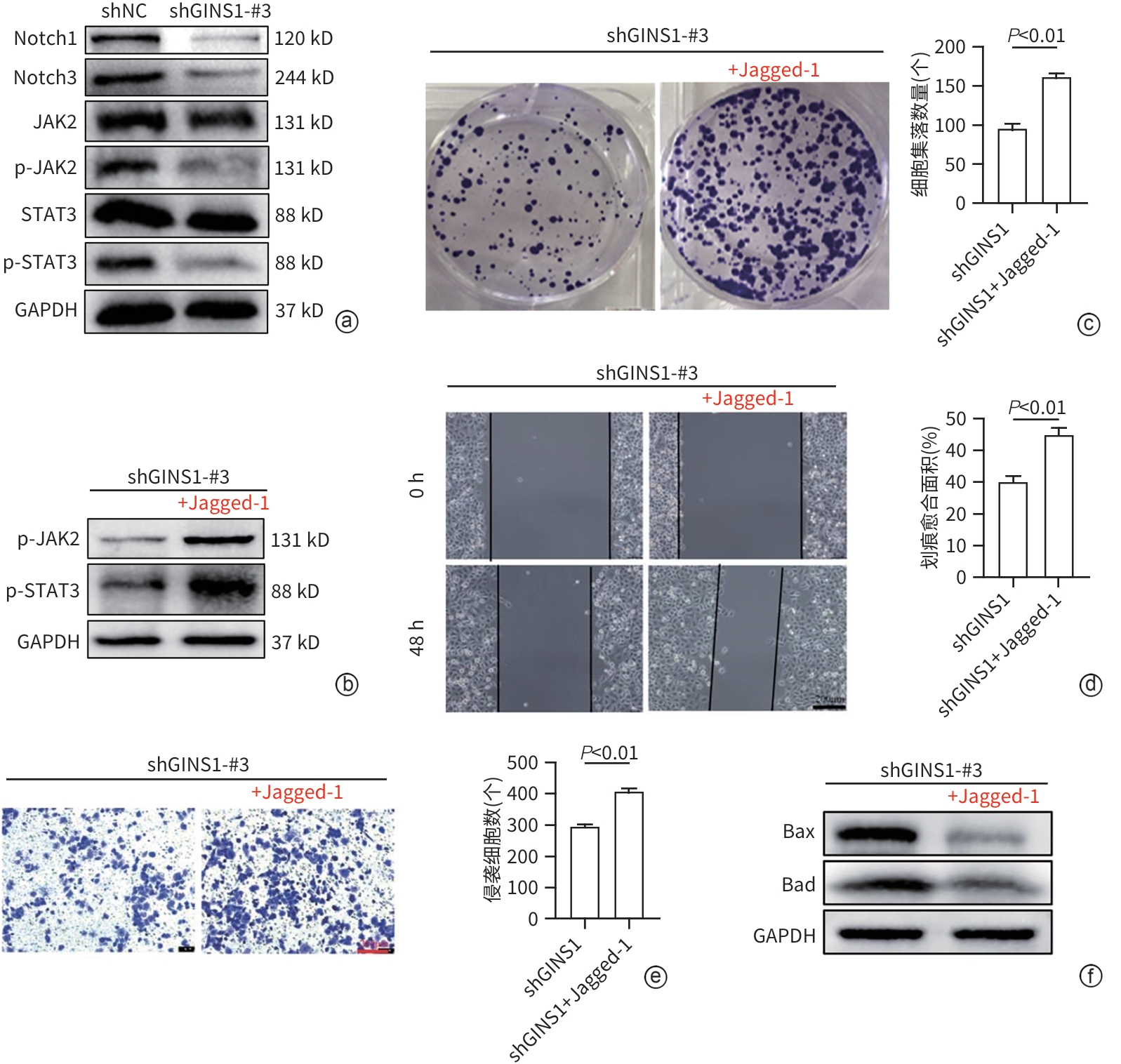
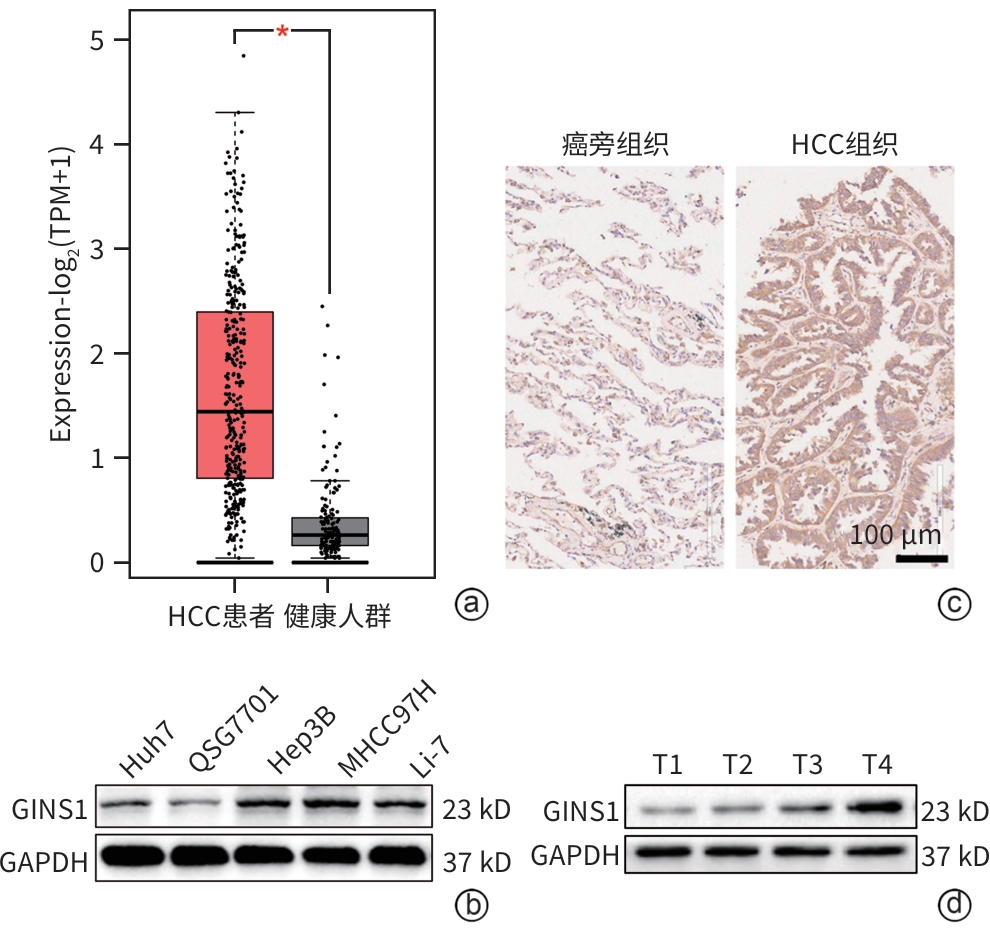
目的 探究Go-ichi-ni-san复合物亚基1(GINS1)在肝细胞癌(HCC)进展和化疗耐药中的作用及相关机制。 方法 通过肿瘤数据库GEPIA2网站检索并分析GINS1在HCC患者和健康人群中的表达差异。收集2017年5月—2021年1月新疆医科大学附属肿瘤医院及第一附属医院收治的40例HCC患者病理组织,通过免疫组化染色检测GINS1在HCC组织和对应癌旁组织中的表达差异,分析GINS1表达水平与HCC临床TNM分期之间的关系。Western Blot检测GINS1在HCC细胞系Huh7、Hep3B、Li-7、MHCC97H和人正常肝细胞系QSG7701中的表达差异。通过慢病毒转染细胞的方法构建稳定敲低GINS1的MHCC97H细胞株及其阴性对照细胞株。通过CCK-8实验和克隆形成实验检测细胞增殖能力,划痕实验检测细胞迁移能力,Transwell实验检测细胞侵袭能力,使用奥沙利铂处理细胞以检测细胞对化疗药物的敏感性。构建裸鼠负瘤模型,观察GINS1敲低对HCC体内生长的影响。Western Blot检测各组细胞Notch通路和JAK/STAT通路蛋白表达水平。加入Notch受体激动剂Jagged-1处理细胞,分析GINS1与Notch/JAK/STAT通路之间的关系。计量资料两组间比较采用成组t检验,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 GINS1在HCC患者、HCC组织和HCC细胞系中均表达上调(P值均<0.05)。GINS1表达水平与HCC临床TNM分期正相关(r=0.822,P=0.011)。与阴性对照组细胞相比,敲低GINS1的MHCC97H细胞增殖、迁移和侵袭活性均降低(P值均<0.01),对奥沙利铂敏感性增强(P<0.01)。与对照组裸鼠相比,GINS1敲低导致裸鼠形成的肿瘤质量和体积均受到显著抑制(P值均<0.001)。与阴性对照组细胞相比,在敲低GINS1的MHCC97H细胞内,Notch1、Notch3、p-JAK2和p-STAT3表达水平明显降低(P值均<0.05),JAK2和STAT3总体表达水平无明显差异(P值均>0.05)。Jagged-1处理后,敲低GINS1的MHCC97H细胞的增殖、迁移和侵袭活性均有所增加,而细胞对奥沙利铂敏感性有所减弱,p-JAK2、p-STAT3水平升高(P值均<0.05)。 结论 GINS1在HCC中表达上调,并且能够通过Notch/JAK2/STAT3通路促进HCC进展和肿瘤细胞化疗耐药。 Abstract:Objective To investigate the role and mechanism of Go-Ichi-Ni-San complex subunit 1 (GINS1) in the progression of hepatocellular carcinoma (HCC) and the development of chemotherapy resistance. Methods The tumor database GEPIA2 was used to analyze the differential expression of GINS1 between HCC patients and healthy individuals, and pathological tissue samples were collected from 40 HCC patients who were admitted to The Affiliated Tumor Hospital of Xinjiang Medical University and the First Affiliated Hospital of Xinjiang Medical University from May 2017 to January 2021. Immunohistochemical staining was used to measure the difference in the expression of GINS1 between HCC tissue and corresponding adjacent tissue, and the correlation between the expression level of GINS1 and the clinical TNM stage of HCC was analyzed. Western blot was also used to measure the difference in the expression of GINS1 between HCC Huh7/Hep3B/Li-7/MHCC97H cell lines and normal human QSG7701 hepatocytes. The method of lentivirus transfection was used to establish the MHCC97H cell line with stable GINS1 knockdown and its negative control cell line. CCK-8 assay and colony formation assay were used to measure cell proliferative capacity; scratch assay was used to measure cell migration ability; Transwell assay was used to measure cell invasion ability; cells were treated with oxaliplatin to measure their sensitivity to chemotherapy drugs. Nude mice were used to establish a tumor-bearing model and observe the effect of GINS1 knockdown on the growth of HCC in vivo. Western Blot was used to measure the expression levels of the proteins associated with the Notch pathway and the JAK/STAT pathway. The cells were treated with the Notch receptor agonist Jagged-1 to analyze the association between GINS1 and the Notch/JAK/STAT pathway. The independent-samples t test was used for comparison of continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results The expression of GINS1 was upregulated in HCC patients, HCC tissue, and HCC cell lines (all P<0.05), and the expression level of GINS1 was positively correlated with the clinical TNM stage of HCC (r=0.822, P=0.011). Compared with the negative control cells, the GINS1-knockdown MHCC97H cells showed significant reductions in proliferation, migration, and invasion activities (all P<0.01) and a significantly enhanced sensitivity to oxaliplatin (P<0.01). Compared with the nude mice in the control group, GINS1 knockdown caused significant inhibition of tumor weight and volume in vivo in nude mice (all P<0.001). Compared with the negative control cells, the GINS1-knockdown MHCC97H cells showed significant reductions in the expression levels of Notch1, Notch3, p-JAK2, and p-STAT3 (all P<0.05), while there were no significant differences in the overall expression levels of JAK2 and STAT3 (P>0.05). After Jagged-1 treatment, the GINS1-knockdown MHCC97H cells showed significant increases in proliferation, migration, and invasion activities and a significant reduction in sensitivity to oxaliplatin, as well as significant increases in the levels of p-JAK2 and p-STAT3 (all P<0.05). Conclusion GINS1 is upregulated in HCC and can promote HCC progression and chemotherapy resistance through the Notch/JAK2/STAT3 pathway. -
注: a,Western Blot检测结果;b~f,加入Notch受体激动剂Jagged-1后,通过Western blot(b)检测Jagged-1对shGINS1-MHCC97H组细胞JAK2/STAT3通路磷酸化水平的影响,克隆形成实验(c)检测细胞增殖活性变化,划痕实验(d)检测细胞迁移能力变化,Transwell实验(e)检测细胞侵袭能力变化(×100),35 μmol/L奥沙利铂培养(f)检测细胞凋亡情况。
图 5 GINS1通过Notch/JAK2/STAT3通路促进HCC进展和奥沙利铂耐药
Figure 5. GINS1 promoted HCC progression and oxaliplatin resistance through the Notch/JAK2/STAT3 pathway
表 1 qRT-PCR引物序列
Table 1. Primer sequences for qRT-PCR
基因 序列(5'-3') GAPDH 上游:CTCACCGGATGCACCAATGTT
下游:CGCGTTGCTCACAATGTTCAT
GINS1 上游:ACGAGGATGGACTCAGACAAG
下游:TGCAGCGTCGATTTCTTAACA
表 2 shRNA序列
Table 2. shRNA sequence
shRNA 序列(5'-3') shGINS1-#1 CCGGCAAGTTCTGGAGGAGATGAAACTCGAGTTTCATCTCCTCCAGAACTTGTTTTTT shGINS1-#2 CCGGGAGGAGATGAAAGCTTTGTATCTCGAGATACAAAGCTTTCATCTCCTCTTTT shGINS1-#3 CCGGACCACTGTTCTCTGTTAAGAACTCGAGTTCTTAACAGAACAGTGTCTTTTTT -
[1] WANG Y, DENG BC. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers[J]. Cancer Metastasis Rev, 2023, 42( 3): 629- 652. DOI: 10.1007/s10555-023-10084-4. [2] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [3] WANG WY, WEI C. Advances in the early diagnosis of hepatocellular carcinoma[J]. Genes Dis, 2020, 7( 3): 308- 319. DOI: 10.1016/j.gendis.2020.01.014. [4] YE Y, SONG YN, HE SF, et al. GINS2 promotes cell proliferation and inhibits cell apoptosis in thyroid cancer by regulating CITED2 and LOXL2[J]. Cancer Gene Ther, 2019, 26( 3-4): 103- 113. DOI: 10.1038/s41417-018-0045-y. [5] SEKEDAT MD, FENYÖ D, ROGERS RS, et al. GINS motion reveals replication fork progression is remarkably uniform throughout the yeast genome[J]. Mol Syst Biol, 2010, 6: 353. DOI: 10.1038/msb.2010.8. [6] OGINO H, ISHINO S, MAYANAGI K, et al. The GINS complex from the thermophilic archaeon, Thermoplasma acidophilum may function as a homotetramer in DNA replication[J]. Extremophiles, 2011, 15( 4): 529- 539. DOI: 10.1007/s00792-011-0383-2. [7] YANG H, LIU XC, ZHU XL, et al. GINS1 promotes the proliferation and migration of glioma cells through USP15-mediated deubiquitination of TOP2A[J]. iScience, 2022, 25( 9): 104952. DOI: 10.1016/j.isci.2022.104952. [8] BAXLEY RM, LEUNG W, SCHMIT MM, et al. Bi-allelic MCM10 variants associated with immune dysfunction and cardiomyopathy cause telomere shortening[J]. Nat Commun, 2021, 12( 1): 1626. DOI: 10.1038/s41467-021-21878-x. [9] XU XL, CHEN E, MO LH, et al. BRCA1 represses DNA replication initiation through antagonizing estrogen signaling and maintains genome stability in parallel with WEE1-MCM2 signaling during pregnancy[J]. Hum Mol Genet, 2019, 28( 5): 842- 857. DOI: 10.1093/hmg/ddy398. [10] AHMAD M, HAMEED Y, KHAN M, et al. Up-regulation of GINS1 highlighted a good diagnostic and prognostic potential of survival in three different subtypes of human cancer[J]. Braz J Biol, 2021, 84: e250575. DOI: 10.1590/1519-6984.250575. [11] FU QQ, ZHENG H, WANG X, et al. GINS1 promotes the initiation and progression of bladder cancer by activating the AKT/mTOR/c-Myc signaling pathway[J]. Exp Cell Res, 2024, 440( 1): 114125. DOI: 10.1016/j.yexcr.2024.114125. [12] WANG KC, WANG MD, YANG T. Key points in AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma(2023)[J]. J Clin Hepatol, 2023, 39( 9): 2081- 2086. DOI: 10.3969/j.issn.1001-5256.2023.09.008.王科淳, 王明达, 杨田.《2023年美国肝病学会实践指导: 肝细胞癌的预防、诊断和治疗》意见要点[J]. 临床肝胆病杂志, 2023, 39( 9): 2081- 2086. DOI: 10.3969/j.issn.1001-5256.2023.09.008. [13] LI Z, ZHU JY. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707.李照, 朱继业.《原发性肝癌诊疗指南(2024年版)》解读[J]. 临床肝胆病杂志, 2024, 40( 7): 1324- 1327. DOI: 10.12449/JCH240707. [14] UENO M, ITOH M, KONG LY, et al. PSF1 is essential for early embryogenesis in mice[J]. Mol Cell Biol, 2005, 25( 23): 10528- 10532. DOI: 10.1128/MCB.25.23.10528-10532.2005. [15] NAGAHAMA Y, UENO M, MIYAMOTO S, et al. PSF1, a DNA replication factor expressed widely in stem and progenitor cells, drives tumorigenic and metastatic properties[J]. Cancer Res, 2010, 70( 3): 1215- 1224. DOI: 10.1158/0008-5472.CAN-09-3662. [16] MAI WH, CHEN CX, LIU QY, et al. Regulation of Notch signaling pathway in immune responses during infection[J]. Chin J Immunol, 2024, 40( 4): 872- 879.麦文豪, 陈楚溪, 刘巧媛, 等. Notch信号通路在感染过程中对免疫应答的调控作用[J]. 中国免疫学杂志, 2024, 40( 4): 872- 879. [17] ZHOU BH, LIN WL, LONG YL, et al. Notch signaling pathway: Architecture, disease, and therapeutics[J]. Signal Transduct Target Ther, 2022, 7( 1): 95. DOI: 10.1038/s41392-022-00934-y. [18] ZHANG XY, SU TH, WU YF, et al. N6-methyladenosine reader YTHDF1 promotes stemness and therapeutic resistance in hepatocellular carcinoma by enhancing NOTCH1 expression[J]. Cancer Res, 2024, 84( 6): 827- 840. DOI: 10.1158/0008-5472.CAN-23-1916. [19] WU WR, SHI XD, ZHANG FP, et al. Activation of the Notch1-c-myc-VCAM1 signalling axis initiates liver progenitor cell-driven hepatocarcinogenesis and pulmonary metastasis[J]. Oncogene, 2022, 41( 16): 2340- 2356. DOI: 10.1038/s41388-022-02246-5. [20] GRAMANTIERI L, GIOVANNINI C, LANZI A, et al. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma[J]. Liver Int, 2007, 27( 7): 997- 1007. DOI: 10.1111/j.1478-3231.2007.01544.x. [21] HU L, XUE F, SHAO MH, et al. Aberrant expression of Notch3 predicts poor survival for hepatocellular carcinomas[J]. Biosci Trends, 2013, 7( 3): 152- 156. [22] GIOVANNINI C, BAGLIONI M, BARON TOALDO M, et al. Notch3 inhibition enhances sorafenib cytotoxic efficacy by promoting GSK3b phosphorylation and p21 down-regulation in hepatocellular carcinoma[J]. Oncotarget, 2013, 4( 10): 1618- 1631. DOI: 10.18632/oncotarget.1221. [23] GIOVANNINI C, SALZANO AM, BAGLIONI M, et al. Brivanib in combination with Notch3 silencing shows potent activity in tumour models[J]. Br J Cancer, 2019, 120( 6): 601- 611. DOI: 10.1038/s41416-018-0375-4. [24] ZHU WH, LIANG Q, YANG X, et al. Combination of sorafenib and Valproic acid synergistically induces cell apoptosis and inhibits hepatocellular carcinoma growth via down-regulating Notch3 and pAkt[J]. Am J Cancer Res, 2017, 7( 12): 2503- 2514. [25] XIN P, XU XY, DENG CJ, et al. The role of JAK/STAT signaling pathway and its inhibitors in diseases[J]. Int Immunopharmacol, 2020, 80: 106210. DOI: 10.1016/j.intimp.2020.106210. [26] YI L, LI WD, WANG Y, et al. Effects of aloperine on proliferation, apoptosis and immune escape of colorectal cancer cells by regulating IL-6/JAK1/STAT3 signaling pathway[J]. Chin J Immunol, 2024, 40( 7): 1436- 1440.伊亮, 李伟东, 王有, 等. 苦豆碱调节IL-6/JAK1/STAT3信号通路对结直肠癌细胞增殖、凋亡和免疫逃逸的影响[J]. 中国免疫学杂志, 2024, 40( 7): 1436- 1440. [27] CARAGLIA M, VITALE G, MARRA M, et al. Alpha-interferon and its effects on signalling pathways within cells[J]. Curr Protein Pept Sci, 2004, 5( 6): 475- 485. DOI: 10.2174/1389203043379378. [28] ZHAO ZW, SONG JJ, TANG BF, et al. CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway[J]. J Exp Clin Cancer Res, 2020, 39( 1): 259. DOI: 10.1186/s13046-020-01769-7. -



 PDF下载 ( 3409 KB)
PDF下载 ( 3409 KB)

 下载:
下载: