微RNA-128-3p、沉默信息调节因子1(SIRT1)和AMP活化蛋白激酶(AMPK)对2型糖尿病合并非酒精性脂肪性肝病的诊断价值
DOI: 10.12449/JCH250310
Diagnostic value of miR-128-3p, SIRT1, and AMPK in patients with type 2 diabetes mellitus comorbid with nonalcoholic fatty liver disease
-
摘要:
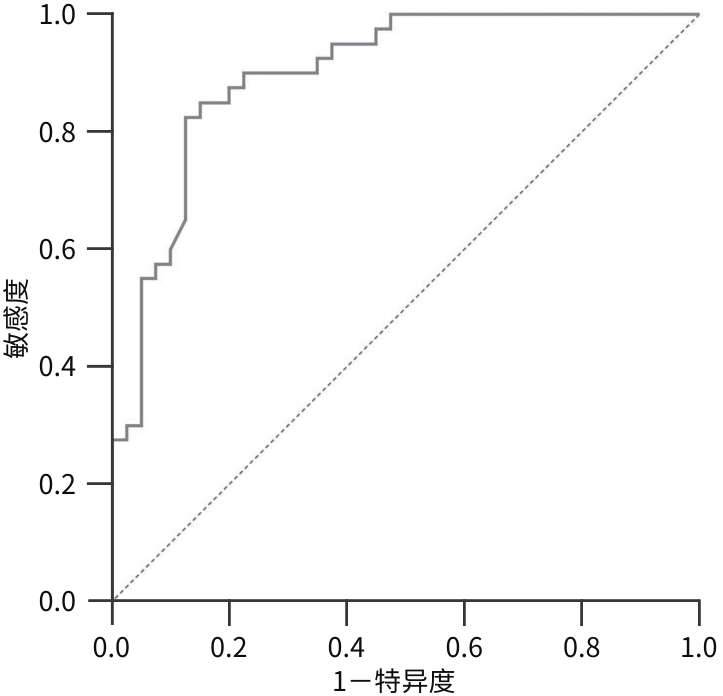
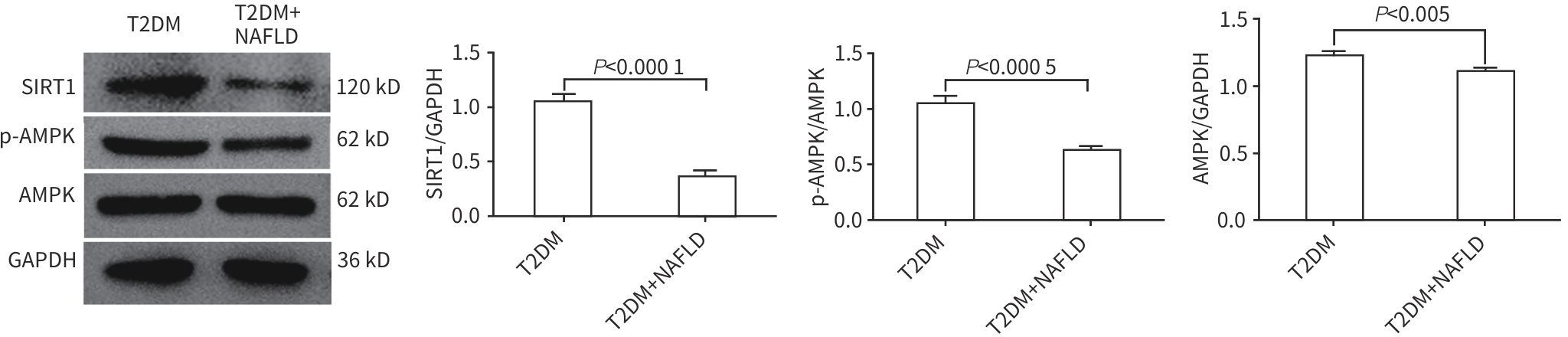
目的 分析2型糖尿病(T2DM)合并非酒精性脂肪性肝病(NAFLD)患者外周血中微RNA(miRNA)-128-3p、沉默信息调节因子1(SIRT1)和AMP活化蛋白激酶(AMPK)的表达情况,探讨miRNA-128-3p对T2DM患者发生NAFLD的预测作用。 方法 选取2022年9月—2023年8月在安徽中医药大学第一附属医院住院的80例T2DM患者,分为T2DM组(40例)和合并NAFLD组(40例),并依据肝纤维化评分(NFS)分为T2DM合并进行性肝纤维化组(16例)和T2DM未合并进行性肝纤维化组(64例),收集基本资料和生化指标,采用定量实时PCR方法检测外周血miRNA-128-3p、SIRT1、AMPK的mRNA表达水平,Western Blot方法检测SIRT1、AMPK蛋白表达水平。正态分布的数据两组间比较采用成组t检验,偏态分布的数据两组间比较采用Mann-Whitney U检验,计数资料两组间比较采用χ2检验;Logistic回归分析NAFLD及进行性肝纤维化的影响因素;使用受试者操作特征曲线(ROC曲线)以确定根据miRNA-128-3p水平判断发生NAFLD的最佳阈值。 结果 合并NAFLD组和T2DM组BMI、空腹血糖、糖化血红蛋白、空腹胰岛素、空腹C肽、ALT、AST、GGT、ALP、纤维连接蛋白、TG、HDL-C、总三碘甲状腺原氨酸(TT3)、胰岛素抵抗指数(HOMA-IR)、NFS比较差异均有统计学意义(P值均<0.05)。合并NAFLD组外周血miRNA-128-3p的mRNA表达水平高于T2DM组(t=-8.765,P<0.001),而SIRT1和AMPK的mRNA及蛋白表达水平均明显降低(P值均<0.001)。T2DM合并进行性肝纤维化组与T2DM未合并进行性肝纤维化组的年龄、ALT、游离三碘甲状腺原氨酸、TT3、超氧化物歧化酶、miRNA-128-3p比较差异均有统计学意义(P值均<0.05)。Logistic回归分析表明,miRNA-128-3p是发生NAFLD和进行性肝纤维化的独立危险因素(OR=8.221,95%CI:2.735~24.714,P<0.001;OR=1.493,95%CI:1.117~1.997,P=0.007);ROC曲线显示其曲线下面积为0.890(95%CI:0.829~0.950),最佳截断值为13.165,敏感度89.3%,特异度72.7%。 结论 miRNA-128-3p在T2DM合并NAFLD患者外周血中表达增高,SIRT1、AMPK表达降低,miRNA-128-3p水平对识别NAFLD及肝纤维化具有一定诊断价值。 -
关键词:
- 糖尿病, 2型 /
- 非酒精性脂肪性肝病 /
- 微RNAs /
- 抗衰老酶1 /
- AMP活化蛋白激酶类
Abstract:Objective To investigate the expression levels of miR-128-3p, SIRT1, and AMPK in the peripheral blood of patients with type 2 diabetes mellitus (T2DM) comorbid with nonalcoholic fatty liver disease (NAFLD), as well as the role of miR-128-3p in predicting NAFLD in T2DM patients. Methods A total of 80 patients with T2DM who were hospitalized in The First Affiliated Hospital of Anhui University of Chinese Medicine from September 2022 to August 2023 were enrolled and divided into T2DM group with 40 patients and NAFLD group with 40 patients, and according to the NAFLD fibrosis score (NFS), the patients were further divided into progressive liver fibrosis group with 16 patients and non-progressive liver fibrosis group with 64 patients. General data and biochemical parameters were collected; quantitative real-time PCR was used to measure the mRNA expression levels of miR-128-3p, SIRT1, and AMPK in peripheral blood, and Western blot was used to measure the protein expression levels of SIRT1 and AMPK. The independent-samples t test was used for comparison of normally distributed data between two groups, and the Mann-Whitney U test was used for comparison of data with skewed distribution between two groups; the chi-square test was used for comparison of categorical data between two groups. The logistic regression analysis was used to identify the influencing factors for the presence of NAFLD and progressive liver fibrosis, and the receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of miR-128-3p for predicting NAFLD. Results There were significant differences between the NAFLD group and the non-NAFLD group in body mass index, fasting plasma glucose, glycated hemoglobin, fasting insulin, fasting C-peptide, alanine aminotransferase (ALT), aspartate aminotransferase, gamma-glutamyl transpeptidase, alkaline phosphatase, fibronectin, triglycerides, high-density lipoprotein cholesterol, total triiodothyronine (TT3), Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), and NFS (all P<0.05). Compared with the non-NAFLD group, the NAFLD group had a significantly higher mRNA expression level of miR-128-3p in peripheral blood (t=-8.765, P<0.001) and significant reductions in the mRNA and proteins expression levels of SIRT1 and AMPK (P<0.001). There were significant differences between the progressive liver fibrosis group and the non-progressive liver fibrosis group in age, ALT, free triiodothyronine, TT3, superoxide dismutase, and miR-128-3p (all P<0.05). The logistic regression analysis showed that miR-128-3p was an independent risk factor for the development of NAFLD (odds ratio [OR]=8.221, 95% confidence interval [CI]: 2.735 — 24.714, P<0.001) and progressive liver fibrosis (OR=1.493, 95%CI: 1.117 — 1.997, P=0.007). The ROC curve analysis showed that miR-128-3p had an area under the ROC curve of 0.890 (95%CI: 0.829 — 0.950), with an optimal cut-off value of 13.165, a sensitivity of 89.3%, and a specificity of 72.7%. Conclusion There is an increase in the expression of miR-128-3p in peripheral blood of T2DM patients with NAFLD, while there are reductions in the expression levels of SIRT1 and AMPK, suggesting that miR-128-3p has a certain diagnostic value in identifying NAFLD and liver fibrosis in such population. -
表 1 Q-PCR引物序列
Table 1. Q-PCR primer sequences
基因 方向 序列(5'-3') 大小(bp) miR-128-3p F CGCTCACAGTGAACCGGTCTCTTT 24 R AGTGCAGGGTCCGAGGTATT 20 SIRT1 F ACGCCTTATCCTCTAGTTCCTGTGTG 26 R GGTCTGTCAGCATCATCTTCCAAG 24 AMPK F GCCTCGCCATACTCTTGATGAGC 23 R TTCTTCCGTCGAACACGCAAGTAG 24 U6 F CTCGCTTCGGCAGCACA 17 R AACGCTTCACGAATTTGCGT 20 GAPDH F CTGCAGACACCTGCCAAGTATG 22 R GCTGCAAGAATGCGAGTTGCT 21 表 2 T2DM组及T2DM合并NAFLD组临床资料的比较
Table 2. Comparison of clinical data in T2DM groups with or without NAFLD
指标 T2DM组(n=40) 合并NAFLD组(n=40) 统计值 P值 年龄(岁) 55.00(46.50~64.25) 57.50(47.75~62.75) Z=-0.313 0.754 男[例(%)] 25(62.50) 24(60.00) χ2=1.507 0.818 BMI(kg/m2) 23.57(22.07~24.89) 25.54(23.69~27.96) Z=-3.729 <0.001 FBG(mmol/L) 6.00(4.96~8.45) 8.17(6.55~10.25) Z=-3.233 0.001 PPG(mmol/L) 11.73(9.69~18.01) 13.89(10.36~17.85) Z=-1.275 0.202 HBA1c(%) 6.90(6.05~8.75) 8.40(7.28~10.06) Z=-3.200 0.001 FINS(μU/mL) 5.88(4.29~8.69) 10.18(6.68~15.30) Z=-3.743 <0.001 FCP(ng/mL) 1.47±0.87 2.61±1.10 t=-5.123 <0.001 ALT(U/L) 14.55(10.20~18.05) 22.45(14.70~29.70) Z=-3.739 <0.001 AST(U/L) 15.95(13.90~18.50) 18.30(16.20~22.48) Z=-3.238 0.001 GGT(U/L) 13.50(12.00~20.75) 26.50(20.25~44.25) Z=-4.770 <0.001 ALP(U/L) 63.50(54.00~75.75) 74.50(62.50~85.75) Z=-2.460 0.014 LDH(U/L) 154.00(144.00~171.50) 168.00(149.50~178.00) Z=-1.078 0.281 ADA(U/L) 6.80(5.75~9.13) 6.30(6.60~10.10) Z=-1.906 0.057 FN(mg/L) 313.65(295.23~339.25) 328.90(315.08~368.90) Z=-2.016 0.048 Cr(μmol/L) 65.92±14.94 60.37±11.87 t=1.840 0.07 UA(μmol/L) 289.50(257.50~375.75) 329.50(275.25~403.25) Z=-1.564 0.118 TG(mmol/L) 1.28(0.91~1.87) 2.36(1.54~3.81) Z=-4.229 <0.001 TC(mmol/L) 4.96±1.28 4.95±1.35 t=0.009 0.993 HDL-C(mmol/L) 1.28(1.01~1.48) 1.05(0.95~1.26) Z=-2.382 0.017 LDL-C(mmol/L) 3.14±0.92 3.02±0.76 t=0.668 0.506 HCY(mg/L) 9.25(7.60~10.65) 9.95(8.43~10.95) Z=-1.429 0.153 RBP(mg/L) 32.56(28.19~42.46) 33.08(29.32~40.96) Z=-0.443 0.658 cysC(mg/L) 0.86(0.76~1.04) 0.89(0.80~1.00) Z=-0.809 0.419 SOD(U/mL) 172.50(166.25~188.00) 178.00(155.25~190.50) Z=-0.515 0.607 ACR(mg/g) 10.73(5.19~48.55) 11.53(6.37~27.44) Z=-0.313 0.754 FT3(pmol/L) 4.09±0.47 4.14±0.58 t=-0.483 0.63 FT4(pmol/L) 12.48(11.86~13.49) 12.65(11.47~13.32) Z=-0.515 0.607 TT3(nmol/L) 1.26±0.21 1.30±0.22 t=-0.685 0.049 TT4(nmol/L) 92.29±14.60 91.49±17.32 t=0.222 0.825 TSH(mIU/L) 1.47(1.08~2.26) 1.78(1.14~2.89) Z=-0.529 0.597 HOMA-IR 2.56(2.36~2.92) 3.68(3.02~4.78) Z=-5.810 <0.001 NFS -0.04±0.81 -0.29±0.97 t=-3.127 0.040 注:ACR,尿白蛋白/尿肌酐比值;FT3,游离三碘甲状腺原氨酸;FT4,游离甲状腺素;TSH,促甲状腺激素。
表 3 T2DM合并与未合并进行性肝纤维化的临床资料及miR-128-3p表达水平的比较
Table 3. Comparison of clinical data and miR-128-3p expression levels in T2DM groups with or without progressive liver fibrosis
指标 T2DM合并进行性肝纤维化
(n=16)
T2DM未合并进行性肝纤维化(n=64) 统计值 P值 年龄(岁) 65.50(60.25~68.00) 53.00(45.99~59.75) Z=-3.853 <0.001 男[例(%)] 8(50.00) 41(60.33) χ2=1.047 0.226 BMI(kg/m2) 24.33(22.77~28.29) 24.50(22.87~25.73) Z=-0.628 0.524 FBG(mmol/L) 7.15(5.49~8.98) 6.66(4.90~12.74) Z=-0.006 0.995 PPG(mmol/L) 13.27(9.97~20.18) 13.10(9.87~17.66) Z=-0.638 0.524 HBA1c(%) 7.22(6.72~9.69) 7.91(6.55~9.50) Z=-0.475 0.635 FINS(μU/mL) 8.69(3.98~14.96) 7.63(5.22~10.87) Z=-0.096 0.923 FCP(ng/mL) 2.07±1.23 2.03±1.12 t=-0.123 0.903 ALT(U/L) 13.95(11.53~17.08) 18.00(13.00~25.48) Z=-2.111 0.035 AST(U/L) 18.25(15.58~22.00) 17.25(14.93~20.63) Z=-0.301 0.764 GGT(U/L) 15.50(13.00~31.25) 21.50(12.25~30.75) Z=-0.801 0.423 ALP(U/L) 56.00(55.50~83.75) 70.00(55.00~82.75) Z=-0.361 0.718 LDH(U/L) 173.50(155.25~184.25) 157.50(144.00~171.50) Z=-1.955 0.051 ADA(U/L) 7.40(6.82~10.53) 7.65(5.83~9.68) Z=-1.011 0.312 FN(mg/L) 316.95(303.13~351.63) 323.30(300.18~351.93) Z=-0.090 0.928 Cr(μmol/L) 63.03±13.95 63.61±13.08 t=0.795 0.880 UA(μmol/L) 321(263.25~374.25) 321(262.25~386.75) Z=-0.102 0.919 TG(mmol/L) 1.53(0.99~2.34) 1.71(1.15~3.16) Z=-1.107 0.268 TC(mmol/L) 5.00±1.31 4.77±1.28 t=0.617 0.539 HDL-C(mmol/L) 1.13(0.95~1.57) 1.14(0.98~1.30) Z=-0.451 0.652 LDL-C(mmol/L) 3.11±0.84 2.97±0.87 t=0.591 0.556 HCY(mg/L) 9.45(8.13~10.40) 9.50(7.75~10.95) Z=-0.337 0.736 RBP(mg/L) 32.70(29.33~43.44) 32.69(29.16~39.93) Z=-0.150 0.880 cysC(mg/L) 0.95(0.82~1.06) 0.87(0.78~0.99) Z=-1.432 0.152 SOD(U/mL) 178.77±15.93 160.56±13.75 t=0.255 <0.001 ACR(mg/g) 15.11(6.14~27.54) 10.93(5.85~38.81) Z=-0.613 0.540 FT3(pmol/L) 4.19±0.50 3.81±0.53 t=0.655 0.008 FT4(pmol/L) 12.69±1.34 12.30±1.00 Z=0.213 0.274 TT3(nmol/L) 1.31±0.20 1.14±0.22 t=0.951 0.005 TT4(nmol/L) 92.20±16.65 90.65±12.98 t=0.393 0.731 TSH(mIU/L) 1.48(1.15~1.90) 1.70(1.08~2.77) Z=-0.529 0.597 HOMA-IR 2.84(2.57~4.07) 3.05(2.46~4.25) Z=-0.325 0.745 miR-128-3p 15.73±1.87 13.13±2.50 t=-4.513 <0.001 表 4 T2DM组及合并NAFLD组miR-128-3p、SIRT1、AMPK表达水平的比较
Table 4. Comparison of the expression levels of miR-128-3p, SIRT1, and AMPK in T2DM groups with or without NAFLD
指标 T2DM组(n=40) 合并NAFLD组(n=40) t值 P值 miR-128-3p 11.37±1.32 15.96±1.11 -8.765 <0.001 SIRT1 13.26±5.23 8.45±3.28 -8.634 <0.001 AMPK 53.50±10.60 41.20±9.37 -10.562 <0.001 表 5 NAFLD相关因素的Logistic回归分析
Table 5. The multivariable Logistic regression analysis for factors associated with NAFLD
变量 β值 SE Wald OR 95%CI P值 miR-128-3p 2.107 0.562 14.074 8.221 2.735~24.714 <0.001 ALT 0.152 0.077 3.873 1.164 1.001~1.353 0.049 常量 -32.663 8.822 13.707 表 6 进行性肝纤维化相关因素的Logistic回归分析
Table 6. The multivariable Logistic regression analysis for factors associated with progressive liver fibrosis
变量 β值 SE Wald OR 95%CI P值 miR-128-3p 0.401 0.148 7.316 1.493 1.117~1.997 0.007 年龄 0.170 0.055 9.582 1.185 1.064~1.319 0.002 SOD -0.065 0.025 7.064 0.937 0.893~0.983 0.008 常量 -6.425 5.578 1.327 -
[1] LI L, LIU DW, YAN HY, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies[J]. Obes Rev, 2016, 17( 6): 510- 519. DOI: 10.1111/obr.12407. [2] WORETA TA, van NATTA ML, LAZO M, et al. Validation of the accuracy of the FAST™ score for detecting patients with at-risk nonalcoholic steatohepatitis(NASH) in a North American cohort and comparison to other non-invasive algorithms[J]. PLoS One, 2022, 17( 4): e0266859. DOI: 10.1371/journal.pone.0266859. [3] CHO EEL, ANG CZ, QUEK J, et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis[J]. Gut, 2023, 72( 11): 2138- 2148. DOI: 10.1136/gutjnl-2023-330110. [4] SANYAL AJ, van NATTA ML, CLARK J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease[J]. N Engl J Med, 2021, 385( 17): 1559- 1569. DOI: 10.1056/NEJMoa2029349. [5] ALON L, CORICA B, RAPARELLI V, et al. Risk of cardiovascular events in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Eur J Prev Cardiol, 2022, 29( 6): 938- 946. DOI: 10.1093/eurjpc/zwab212. [6] BISACCIA G, RICCI F, KHANJI MY, et al. Cardiovascular morbidity and mortality related to non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Curr Probl Cardiol, 2023, 48( 6): 101643. DOI: 10.1016/j.cpcardiol.2023.101643. [7] WANG L, SINNOTT-ARMSTRONG N, WAGSCHAL A, et al. A MicroRNA linking human positive selection and metabolic disorders[J]. Cell, 2020, 183( 3): 684- 701. e 14. DOI: 10.1016/j.cell.2020.09.017. [8] ZHAO XR, JIN Y, LI L, et al. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1[J]. Pharmacol Res, 2019, 146: 104276. DOI: 10.1016/j.phrs.2019.104276. [9] SHI RF, JIN YP, HU WW, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy[J]. Am J Physiol Cell Physiol, 2020, 318( 5): C848- C856. DOI: 10.1152/ajpcell.00041.2020. [10] HAN JN, HAO WJ, MA YP, et al. MiR-128-3p promotes the progression of deep venous thrombosis through binding SIRT1[J]. Phlebology, 2023, 38( 8): 540- 549. DOI: 10.1177/02683555231190268. [11] Diabetes Society of Chinese Medical Association. Guideline for the prevention and treatment of type 2 diabetes mellitus in China(2020 edition)[J]. Chin J Diabetes, 2021, 13( 4): 315- 409. DOI: 10.3760/cma.j.cn115791-20210307-00135.中华医学会糖尿病学分会. 中国2型糖尿病防治指南(2020年版)[J]. 中华糖尿病杂志, 2021, 13( 4): 315- 409. DOI: 10.3760/cma.j.cn115791-20210307-00135. [12] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association, Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [13] WANG XS, JIANG LJ, SHAO XN. Association analysis of insulin resistance and osteoporosis risk in Chinese patients with T2DM[J]. Ther Clin Risk Manag, 2021, 17: 909- 916. DOI: 10.2147/TCRM.S328510. [14] ANGULO P, HUI JM, MARCHESINI G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD[J]. Hepatology, 2007, 45( 4): 846- 854. DOI: 10.1002/hep.21496. [15] TARGHER G, COREY KE, BYRNE CD, et al. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 9): 599- 612. DOI: 10.1038/s41575-021-00448-y. [16] STEFAN N, CUSI K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes[J]. Lancet Diabetes Endocrinol, 2022, 10( 4): 284- 296. DOI: 10.1016/S2213-8587(22)00003-1. [17] SONG SJ, LAI JCT, WONG GLH, et al. Can we use old NAFLD data under the new MASLD definition?[J]. J Hepatol, 2024, 80( 2): e54- e56. DOI: 10.1016/j.jhep.2023.07.021. [18] MANTOVANI A, CSERMELY A, TAVERNA A, et al. Association between metabolic dysfunction-associated fatty liver disease and supraventricular and ventricular tachyarrhythmias in patients with type 2 diabetes[J]. Diabetes Metab, 2023, 49( 2): 101416. DOI: 10.1016/j.diabet.2022.101416. [19] NI XT, TONG C, HALENGBIEKE A, et al. Association between nonalcoholic fatty liver disease and type 2 diabetes: A bidirectional two-sample Mendelian randomization study[J]. Diabetes Res Clin Pract, 2023, 206: 110993. DOI: 10.1016/j.diabres.2023.110993. [20] ISMAIL MH, ARGAN R AL, ELAMIN Y, et al. Automated fibrosis-4 index: Simplifying non-alcoholic fatty liver disease for diabetologists[J]. Medicina(Kaunas), 2024, 60( 8): 1278. DOI: 10.3390/medicina60081278. [21] O’CONNELL RM, RAO DS, BALTIMORE D. MicroRNA regulation of inflammatory responses[J]. Annu Rev Immunol, 2012, 30: 295- 312. DOI: 10.1146/annurev-immunol-020711-075013. [22] UDDIN A, CHAKRABORTY S. Role of miRNAs in lung cancer[J]. J Cell Physiol, 2018. DOI: 10.1002/jcp.26607.[ Online ahead of print] [23] YANG JN, JIANG TL, ZHU FB, et al. Research progress in effect of miRNA on podocyte injury in diabetic nephropathy and its mechanism[J]. J Jilin Univ(Med Edit), 2023, 49( 6): 1677- 1682. DOI: 10.13481/j.1671-587X.20230637.杨佳楠, 姜同连, 朱福彬, 等. miRNA在糖尿病肾病足细胞损伤中作用及其机制的研究进展[J]. 吉林大学学报(医学版), 2023, 49( 6): 1677- 1682. DOI: 10.13481/j.1671-587X.20230637. [24] WEI HF, NI ZQ, WEI YH, et al. Effects of miR-126 over-expression and ADAM9 gene silencing on biological behavior of gastric cancer SGC-7901 cells and their mechanisms[J]. J Jilin Univ(Med Edit), 2024, 50( 2): 310- 319. DOI: 10.13481/j.1671-587X.20240203.魏海峰, 倪志强, 魏雁虹, 等. MiR-126过表达和ADAM9基因沉默对胃癌SGC-7901细胞生物学行为的影响及其机制[J]. 吉林大学学报(医学版), 2024, 50( 2): 310- 319. DOI: 10.13481/j.1671-587X.20240203. [25] SUN T, WANG C, HUO L, et al. Serum cortistatin level in type 2 diabetes mellitus and its relationship with nonalcoholic fatty liver disease[J]. Int J Gen Med, 2023, 16: 631- 639. DOI: 10.2147/IJGM.S396315. [26] HIRANO T, SATOH N, ITO Y. Specific increase in small dense low-density lipoprotein-cholesterol levels beyond triglycerides in patients with diabetes: Implications for cardiovascular risk of MAFLD[J]. J Atheroscler Thromb, 2024, 31( 1): 36- 47. DOI: 10.5551/jat.64271. [27] BI TB. Relationship between thyroid hormone levels and metabolic dysfunction associated steatotic liver disease in patients with type 2 diabetes: A clinical study[J]. Medicine(Baltimore), 2024, 103( 26): e38643. DOI: 10.1097/MD.0000000000038643. [28] ZHANG XD, CHEN YM, YE HY, et al. Correlation between thyroid function, sensitivity to thyroid hormones and metabolic dysfunction-associated fatty liver disease in euthyroid subjects with newly diagnosed type 2 diabetes[J]. Endocrine, 2023, 80( 2): 366- 379. DOI: 10.1007/s12020-022-03279-2. [29] HEIANZA Y, XUE QC, ROOD J, et al. Circulating thrifty microRNA is related to insulin sensitivity, adiposity, and energy metabolism in adults with overweight and obesity: The POUNDS lost trial[J]. Am J Clin Nutr, 2023, 117( 1): 121- 129. DOI: 10.1016/j.ajcnut.2022.10.001. [30] CHANG E. Vitamin D mitigates hepatic fat accumulation and inflammation and increases SIRT1/AMPK expression in AML-12 hepatocytes[J]. Molecules, 2024, 29( 6): 1401. DOI: 10.3390/molecules29061401. [31] XIAO Q, ZHANG SJ, YANG C, et al. Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in HepG2 cells via the AMPK/NF-κB pathway[J]. Int J Endocrinol, 2019, 2019: 7514802. DOI: 10.1155/2019/7514802. [32] WU LZ, ZHANG GR, GUO CB, et al. MiR-128-3p mediates TNF-α-induced inflammatory responses by regulating Sirt1 expression in bone marrow mesenchymal stem cells[J]. Biochem Biophys Res Commun, 2020, 521( 1): 98- 105. DOI: 10.1016/j.bbrc.2019.10.083. [33] SAMY AM, KANDEIL MA, SABRY D, et al. Exosomal miR-122, miR-128, miR-200, miR-298, and miR-342 as novel diagnostic biomarkers in NAFL/NASH: Impact of LPS/TLR-4/FoxO3 pathway[J]. Arch Pharm(Weinheim), 2024, 357( 4): e2300631. DOI: 10.1002/ardp.202300631. -



 PDF下载 ( 1188 KB)
PDF下载 ( 1188 KB)

 下载:
下载: