晚期肝细胞癌的系统治疗
DOI: 10.12449/JCH241127
-
摘要: 肝细胞癌是全球范围内发病率和死亡率前列的恶性肿瘤。随着分子生物学和肿瘤免疫学的进步,以酪氨酸激酶抑制剂(如索拉非尼、仑伐替尼等)为代表的分子靶向药物和以程序性死亡受体-1/程序性死亡配体-1单抗为代表的免疫治疗为晚期肝细胞癌患者带来福音。免疫治疗联合抗血管生成治疗可进一步提高疗效。此外,立体定向放疗、局部治疗与系统治疗的优化整合有望使得患者获益最大化。未来,深入理解肝细胞癌异质性,发展精准分子分型和个体化治疗,建立多学科协作诊疗体系,系统治疗有望实现晚期肝细胞癌的长期管理。本文就晚期肝细胞癌系统治疗的研究现状和进展作一综述。Abstract: Hepatocellular carcinoma (HCC) is one of the most common malignancies with high morbidity and mortality rates worldwide. With the advances in molecular biology and tumor immunology, molecular-targeted agents represented by tyrosine kinase inhibitors (such as sorafenib and lenvatinib) and immunotherapy represented by PD-1/PD-L1 monoclonal antibodies have brought hope for patients with advanced HCC. The combination of immunotherapy and anti-angiogenic therapy can further improve the treatment outcome of patients. In addition, the optimization and integration of stereotactic body radiotherapy, local treatment, and systemic treatment may maximize the benefits of patients. In the future, through a deep understanding of the heterogeneity of HCC, the development of precision molecular subtyping and individualized treatment, and the establishment of a multidisciplinary collaborative diagnosis and treatment system, systemic therapy is expected to achieve long-term management of advanced HCC. This article reviews the current status and advances in systemic therapy for advanced HCC.
-
Key words:
- Carcinoma, Hepatocellular /
- Precision Medicine /
- Therapeutics
-
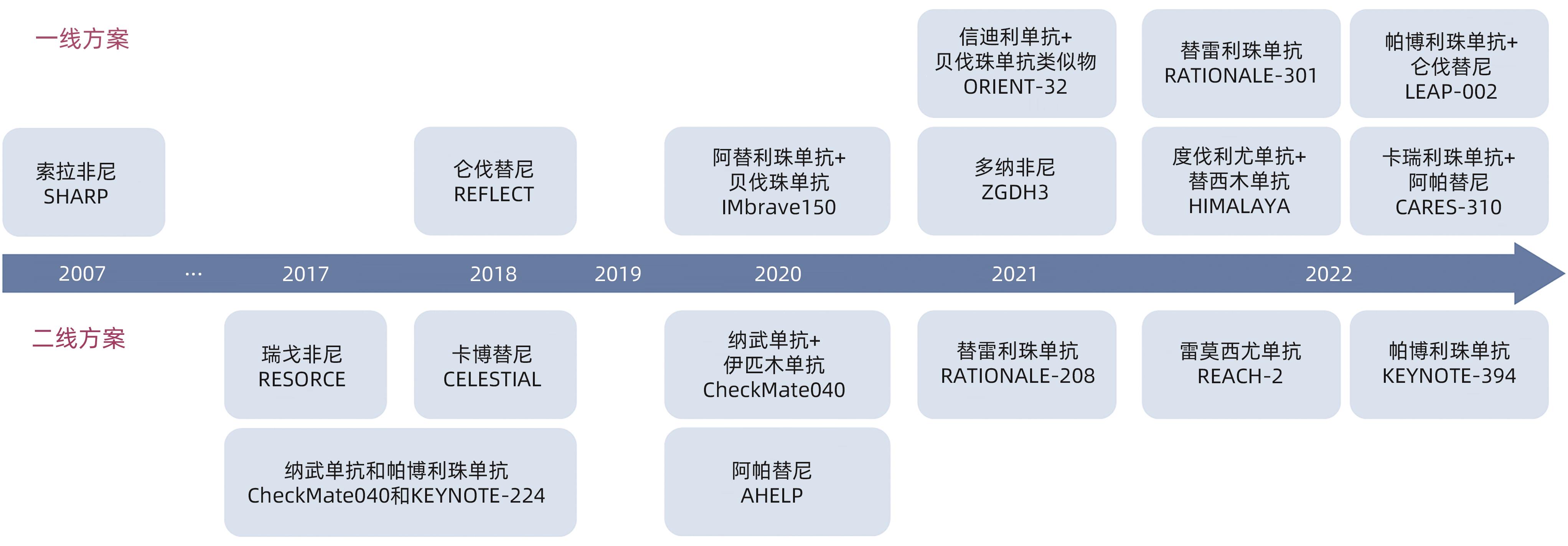
表 1 ICI联合治疗在晚期HCC一线治疗中的Ⅲ期临床研究结果汇总
Table 1. Summary of phase Ⅲ clinical trial results of ICI-combined regimen in advanced HCC first-line treatment
类别 IO+大分子单抗 IO+小分子TKI IO+IO IMbrave 150[24] ORIENT-32[29] SHR-1210-Ⅲ-310[30] LEAP-002[31] COSMIC-312[33] HIMALAYA[36] ICI药物种类 PD-L1抑制剂 PD-1抑制剂 PD-1抑制剂 PD-1抑制剂 PD-L1抑制剂 PD-L1、CTLA-4
抑制剂
药物 阿替利珠单抗+贝伐珠单抗 vs
索拉非尼
信迪利单抗+ IBI305 vs 索拉非尼 卡瑞利珠单抗+阿帕替尼 vs 索拉非尼 帕博利珠单抗+仑伐替尼 vs 仑伐替尼 阿替利珠单抗+卡博替尼 vs 索拉非尼 度伐利尤单抗+替西木单抗 vs 索拉非尼 研究设计 全球、多中心、开放标签、随机对照,Ⅲ期 多中心、开放标签、随机对照、Ⅱ/Ⅲ期 全球、随机、开放标签、Ⅲ期 全球、随机、双盲、安慰剂对照、Ⅲ期 全球多中心、开放标签、随机、Ⅲ期 全球、多中心、开放标签、随机对照,Ⅲ期 入组人数(例) 501 571 543 794 837 782 特有入组标准 内镜检查,排除出血或高出血风险 Child-Pugh评分≤7分 随机化后3个月内的内静检查;无门静脉主干侵犯(Vp4) 无门静脉主干
侵犯(Vp4)
中位OS(月) 19.2 vs 13.4
HR=0.66
NR vs 10.4
HR=0.57
22.1 vs 15.2
HR=0.62
21.2 vs 19.0
HR=0.840
15.4 vs 15.5
HR=0.90
16.4 vs 13.8
HR=0.78
中位PFS(月) 6.9 vs 4.3
HR=0.65
4.6 vs 2.8
HR=0.56
5.6 vs 3.7
HR=0.52
8.2 vs 8.1
HR=0.834
6.8 vs 4.2
HR=0.63
3.78 vs 4.07
HR=0.90
ORR 30% vs 11% 21% vs 4% 25.4% vs 5.9% 26.1% vs 17.5% 11% vs 4% 20.1% vs 5.1% DCR 74% vs 55% 72% vs 64% 78.3% vs 53.9% 81.3% vs 78.4% 78% vs 65% 60.1% vs 60.7% PD 19% vs 25% 27% vs 33% 16.2% vs 36.5% 12.2% vs 15.0% 14% vs 20% 20.6% vs 6.7% 任何级别AE发生率 86% vs 95% 89% vs 94% 96.5% vs 95.7% 93% vs 90% 76% vs 85% ≥3级AE发生率 45% vs 47% 34% vs 36% 81% vs 52% 63% vs 58% 55% vs 33% 26% vs 37% 所有级别出血发生率 25% vs17.3% 4.7% vs 4.9% 1% vs 1% 1.8% vs 4.8% G3/4出血发生率 6.3% vs 5.8% 3.4% vs 2.7% 0.5% vs 1% 0.5% vs 1.6% 注:IO,肿瘤免疫疗法;PD,疾病发展;NR,未达到。
表 2 正在进行的局部联合系统治疗不可切除HCC的Ⅲ期临床研究
Table 2. Ongoing phase Ⅲ clinical trials of locoreginal therapy combined with systemic therapy in patients with unresectable HCC
研究编码 研究状态 研究类型 入组人数(例) 干预措施 主要终点 入组条件 NCT03778957
(EMERALD-1)
活跃,未招募 Ⅲ期 RCT 600 TACE+度伐利尤单抗+安慰剂;
TACE+度伐利尤单抗+贝伐珠单抗;
TACE+安慰剂+安慰剂
PFS 适用于TACE,Child-Pugh≤7分,PS 0/1分 NCT05301842
(EMERALD-3)
招募中 Ⅲ期 RCT 725 TACE+曲美木单抗+度伐利尤单抗+仑伐替尼;
TACE+曲美木单抗+度伐利尤单抗;
TACE
PFS 适用于TACE,Child-Pugh A级,
PS 0/1分
NCT04246177
(LEAP-012)
活跃,未招募 Ⅲ期 RCT 450 TACE+仑伐替尼+帕博利珠单抗;
TACE
OS和PFS 不适用于根治性治疗 NCT04268888 招募中 Ⅱ/Ⅲ期 RCT 522 TACE+纳武利尤单抗;
TACE/TAE
OS和TTTP 不适用于外科切除或肝移植,
Child-Pugh≤6分
NCT04712643 活跃,未招募 Ⅲ期 RCT 342 TACE+阿替利珠单抗+贝伐珠单抗;
TACE
OS和PFS 适用于TACE,Child-Pugh A级,
PS 0/1分,无PVTT
NCT05738616 招募中 Ⅲ期 RCT 168 TACE+卡瑞利珠单抗+仑伐替尼;
TACE
CRR和OS BCLC C,Child-Pugh A/B级,
PS 0/1分,初治
NCT05320692 招募中 Ⅲ期RCT 360 TACE+卡瑞利珠单抗+阿帕替尼;
TACE
PFS Child-Pugh A级,PS 0/1分 NCT05608213 招募中 Ⅲ期RCT 183 仑伐替尼+I-125放射性粒子植入;
仑伐替尼
OS 由JSH判定TACE 抵抗,Child-Pugh A/B级,PS 0/1分,无PVTT NCT05608200 招募中 Ⅲ期 RCT 427 仑伐替尼+信迪利单抗+TACE;
仑伐替尼+TACE
OS BCLC C,或CNL C Ⅲa and Ⅲb,Child-Pugh A级,PS 0/1分,初治,无PVTT NCT05985798 招募中 Ⅲ期 RCT 258 信迪利单抗+贝伐珠单抗+TACE;
仑伐替尼+TACE
OS BCLC C,Child-Pugh A级,
PS 0/1分,初治,无PVTT
NCT04387695 招募中 Ⅲ期 RCT 54 序贯SBRT+TACE+索拉非尼;
索拉非尼
PFS BCLC C,合并PVTT,
Child-Pugh≤7分,PS≤2分,初治
NCT05313282 招募中 Ⅲ期 RCT 140 mFOLFOX7-HAIC+阿帕替尼+卡瑞利珠单抗;阿帕替尼+卡瑞利珠单抗 PFS BCLC C,Child-Pugh A级,
PS 0/1分,初治
NCT05198609 招募中 Ⅲ期 RCT 214 mFOLFOX7-HAIC+阿帕替尼+卡瑞利珠单抗;阿帕替尼+卡瑞利珠单抗 OS 合并PVTT,初治 -
[1] RUMGAY H, ARNOLD M, FERLAY J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040[J]. J Hepatol, 2022, 77( 6): 1598- 1606. DOI: 10.1016/j.jhep.2022.08.021. [2] YANG T, WANG MD, XU XF, et al. Management of hepatocellular carcinoma in China: Seeking common grounds while reserving differences[J]. Clin Mol Hepatol, 2023, 29( 2): 342- 344. DOI: 10.3350/cmh.2023.0106. [3] YANG T, ZHANG H, LAU WY, et al. Liver disease in China: A long way to go[J]. Hepatology, 2015, 62( 5): 1640. DOI: 10.1002/hep.27769. [4] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380( 15): 1450- 1462. DOI: 10.1056/nejmra1713263. [5] WANG MD, DIAO YK, YAO LQ, et al. Emerging role of molecular diagnosis and personalized therapy for hepatocellular carcinoma[J]. iLIVER, 2024, 3( 1): 100083. DOI: 10.1016/j.iliver.2024.100083. [6] YANG C, ZHANG HL, ZHANG LM, et al. Evolving therapeutic landscape of advanced hepatocellular carcinoma[J]. Nat Rev Gastroenterol Hepatol, 2023, 20( 4): 203- 222. DOI: 10.1038/s41575-022-00704-9. [7] ZHU AX, DUDA DG, SAHANI DV, et al. HCC and angiogenesis: Possible targets and future directions[J]. Nat Rev Clin Oncol, 2011, 8( 5): 292- 301. DOI: 10.1038/nrclinonc.2011.30. [8] LLOVET JM, RICCI S, MAZZAFERRO V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. N Engl J Med, 2008, 359( 4): 378- 390. DOI: 10.1056/NEJMoa0708857. [9] CHENG AL, KANG YK, CHEN ZD, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10( 1): 25- 34. DOI: 10.1016/S1470-2045(08)70285-7. [10] KUDO M, FINN RS, QIN SK, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial[J]. Lancet, 2018, 391( 10126): 1163- 1173. DOI: 10.1016/S0140-6736(18)30207-1. [11] ABOU-ALFA GK, MEYER T, CHENG AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma[J]. N Engl J Med, 2018, 379( 1): 54- 63. DOI: 10.1056/NEJMoa1717002. [12] BRUIX J, QIN SK, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment(RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389( 10064): 56- 66. DOI: 10.1016/S0140-6736(16)32453-9. [13] QIN SK, LI Q, GU SZ, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma(AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6( 7): 559- 568. DOI: 10.1016/S2468-1253(21)00109-6. [14] QIN SK, BI F, GU SZ, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: A randomized, open-label, parallel-controlled phase II-III trial[J]. J Clin Oncol, 2021, 39( 27): 3002- 3011. DOI: 10.1200/JCO.21.00163. [15] PINTER M, SCHEINER B, PECK-RADOSAVLJEVIC M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups[J]. Gut, 2021, 70( 1): 204- 214. DOI: 10.1136/gutjnl-2020-321702. [16] LLOVET JM, MONTAL R, SIA D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma[J]. Nat Rev Clin Oncol, 2018, 15( 10): 599- 616. DOI: 10.1038/s41571-018-0073-4. [17] EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma(CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389( 10088): 2492- 2502. DOI: 10.1016/S0140-6736(17)31046-2. [18] KUDO M, MATILLA A, SANTORO A, et al. Checkmate-040: Nivolumab(NIVO) in patients(pts) with advanced hepatocellular carcinoma(aHCC) and Child-Pugh B(CPB) status[J]. J Clin Oncol, 2019, 37( 4_suppl): 327. DOI: 10.1200/jco.2019.37.4_suppl.327. [19] QIN SK, KUDO M, MEYER T, et al. Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: A phase 3 randomized clinical trial[J]. JAMA Oncol, 2023, 9( 12): 1651- 1659. DOI: 10.1001/jamaoncol.2023.4003. [20] ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib(KEYNOTE-224): A non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19( 7): 940- 952. DOI: 10.1016/S1470-2045(18)30351-6. [21] FINN RS, RYOO BY, MERLE P, et al. Pembrolizumab As second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial[J]. J Clin Oncol, 2020, 38( 3): 193- 202. DOI: 10.1200/JCO.19.01307. [22] QIN SK, CHEN ZD, LIU Y, et al. A phase II study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer[J]. J Clin Oncol, 2019, 37( 15_suppl): 4074. DOI: 10.1200/jco.2019.37.15_suppl.4074. [23] FINN RS, IKEDA M, ZHU AX, et al. Phase Ⅰb study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma[J]. J Clin Oncol, 2020, 38( 26): 2960- 2970. DOI: 10.1200/JCO.20.00808. [24] FINN RS, QIN SK, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382( 20): 1894- 1905. DOI: 10.1056/NEJMoa1915745. [25] National Health Commission of the People’s Republic of China. Standard for diagnosis and treatment of primary liver cancer(2024 edition)[J]. J Clin Hepatol, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508.中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2024年版)[J]. 临床肝胆病杂志, 2024, 40( 5): 893- 918. DOI: 10.12449/JCH240508. [26] National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology(NCCN Guidelines). Hepatocellular carcinoma. Version 2. 2024[EB/OL]. https://www.nccn.org/login?ReturnURL=tts://www.nccn.org/professionals/physician%20gls/pdf/hcc.pdf. https://www.nccn.org/login?ReturnURL=tts://www.nccn.org/professionals/physician%20gls/pdf/hcc.pdf [27] SINGAL AG, LLOVET JM, YARCHOAN M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma[J]. Hepatology, 2023, 78( 6): 1922- 1965. DOI: 10.1097/HEP.0000000000000466. [28] HUANG DX, CHEN Y, ZENG QL, et al. Blood supply characteristics of pedunculated hepatocellular carcinoma prior to and following transcatheter arterial chemoembolization treatment: An angiographic demonstration[J]. Oncol Lett, 2018, 15( 3): 3383- 3389. DOI: 10.3892/ol.2018.7844. [29] REN ZG, XU JM, BAI YX, et al. Sintilimab plus a bevacizumab biosimilar(IBI305) versus sorafenib in unresectable hepatocellular carcinoma(ORIENT-32): A randomised, open-label, phase 2-3 study[J]. Lancet Oncol, 2021, 22( 7): 977- 990. DOI: 10.1016/S1470-2045(21)00252-7. [30] QIN SK, CHAN SL, GU SZ, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma(CARES-310): A randomised, open-label, international phase 3 study[J]. Lancet, 2023, 402( 10408): 1133- 1146. DOI: 10.1016/S0140-6736(23)00961-3. [31] LLOVET JM, KUDO M, MERLE P, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma(LEAP-002): A randomised, double-blind, phase 3 trial[J]. Lancet Oncol, 2023, 24( 12): 1399- 1410. DOI: 10.1016/S1470-2045(23)00469-2. [32] QIN SK, XUR R, PAN HM, et al. First-line lenvatinib±pembrolizumab for advanced hepatocellular carcinoma: LEAP-002 China subgroup. APASL 2024, Abstr 100797[EB/OL]. https://www.apasl2024kyoto.org/docs/info/Accepted_Regular_Abstracts-APASL_2024_Kyoto-as_of_2023_12_18.pdf. https://www.apasl2024kyoto.org/docs/info/Accepted_Regular_Abstracts-APASL_2024_Kyoto-as_of_2023_12_18.pdf [33] KELLEY RK, RIMASSA L, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma(COSMIC-312): a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23( 8): 995- 1008. DOI: 10.1016/S1470-2045(22)00326-6. [34] YAU T, KANG YK, KIM TY, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial[J]. JAMA Oncol, 2020, 6( 11): e204564. DOI: 10.1001/jamaoncol.2020.4564. [35] GALLE PR, DECAENS T, KUDO M, et al. Nivolumab(NIVO) plus ipilimumab(IPI) vs lenvatinib(LEN) or sorafenib(SOR) as first-line treatment for unresectable hepatocellular carcinoma(uHCC): First results from CheckMate 9DW[J]. J Clin Oncol, 2024, 42( 17_suppl): LBA4008. DOI: 10.1200/JCO.2024.42.17_suppl.LBA4008. [36] KELLEY RK, SANGRO B, HARRIS W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study[J]. J Clin Oncol, 2021, 39( 27): 2991- 3001. DOI: 10.1200/JCO.20.03555. [37] ABOU-ALFA GK, LAU G, KUDO M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma[J]. NEJM Evid, 2022, 1( 8): EVIDoa2100070. DOI: 10.1056/EVIDoa2100070. [38] KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation(TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69( 8): 1492- 1501. DOI: 10.1136/gutjnl-2019-318934. [39] PENG Z, FAN W, ZHU B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase Ⅲ, randomized clinical trial(LAUNCH)[J]. J Clin Oncol, 2023, 41( 1): 117- 127. DOI: 10.1200/JCO.22.00392. [40] ZHAO M, LYU N, ZHONG S, et al. 983P Safety and efficacy of durvalumab plus hepatic artery infusion chemotherapy in HCC with severe portal vein tumor thrombosis(Vp3/4)-the DurHope study[J]. Ann Oncol, 2023, 34: S608. DOI: 10.1016/j.annonc.2023.09.2128. [41] DAWSON LA, WINTER KA, KNOX JJ, et al. NRG/RTOG 1112: Randomized phase III study of sorafenib vs. stereotactic body radiation therapy(SBRT) followed by sorafenib in hepatocellular carcinoma(HCC)[J]. J Clin Oncol, 2023, 41( 4_suppl): 489. DOI: 10.1200/jco.2023.41.4_suppl.489. [42] YANG X, HU Y, YANG KY, et al. Cell-free DNA copy number variations predict efficacy of immune checkpoint inhibitor-based therapy in hepatobiliary cancers[J]. J Immunother Cancer, 2021, 9( 5): e001942. DOI: 10.1136/jitc-2020-001942. [43] ZAPPASODI R, WOLCHOK JD, MERGHOUB T. Strategies for predicting response to checkpoint inhibitors[J]. Curr Hematol Malig Rep, 2018, 13( 5): 383- 395. DOI: 10.1007/s11899-018-0471-9. [44] REN ZG, GUO YB, BAI YX, et al. Tebotelimab, a PD-1/LAG-3 bispecific antibody, in patients with advanced hepatocellular carcinoma who had failed prior targeted therapy and/or immunotherapy: An open-label, single-arm, phase 1/2 dose-escalation and expansion study[J]. J Clin Oncol, 2023, 41( 4_suppl): 578. DOI: 10.1200/jco.2023.41.4_suppl.578. [45] XING BC, DA X, ZHANG YQ, et al. A phase II study combining KN046(an anti-PD-L1/CTLA-4 bispecific antibody) and lenvatinib in the treatment for advanced unresectable or metastatic hepatocellular carcinoma(HCC): Updated efficacy and safety results[J]. J Clin Oncol, 2022, 40( 16_suppl): 4115. DOI: 10.1200/jco.2022.40.16_suppl.4115. [46] FINN RS, RYOO BY, HSU CH, et al. Results from the MORPHEUS-liver study: Phase Ib/II randomized evaluation of tiragolumab(tira) in combination with atezolizumab(atezo) and bevacizumab(bev) in patients with unresectable, locally advanced or metastatic hepatocellular carcinoma(uHCC)[J]. J Clin Oncol, 2023, 41( 16_suppl): 4010. DOI: 10.1200/jco.2023.41.16_suppl.4010. [47] REN Z, HUANG Y, GUO Y, et al. 945MO AdvanTIG-206: Phase II randomized open-label study of ociperlimab(OCI)+tislelizumab(TIS)+BAT1706(bevacizumab biosimilar) versus TIS+BAT1706 in patients(pts) with advanced hepatocellular carcinoma(HCC)[J]. Ann Oncol, 2023, 34: S594. DOI: 10.1016/j.annonc.2023.09.2091. [48] LU MY, WILLIAMSON DFK, CHEN TY, et al. Data-efficient and weakly supervised computational pathology on whole-slide images[J]. Nat Biomed Eng, 2021, 5( 6): 555- 570. DOI: 10.1038/s41551-020-00682-w. -



 PDF下载 ( 1021 KB)
PDF下载 ( 1021 KB)

 下载:
下载:


