不可逆电穿孔联合免疫治疗对不可切除胰腺癌有效性和安全性的Meta分析
DOI: 10.12449/JCH241122
Efficacy and safety of irreversible electroporation combined with immunotherapy in treatment of unresectable pancreatic cancer: A Meta-analysis
-
摘要:
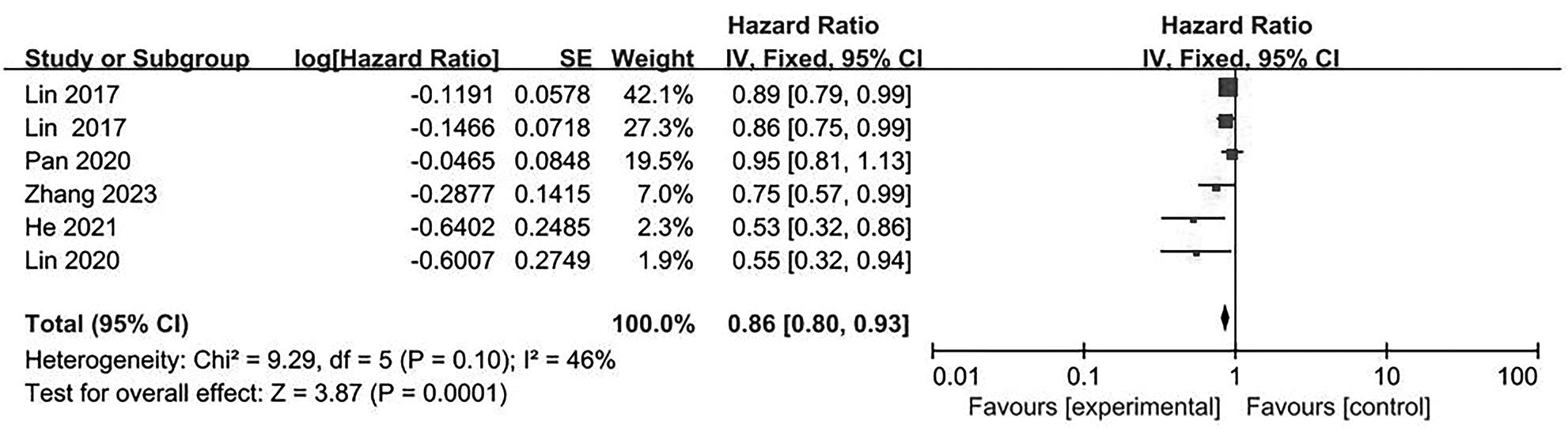
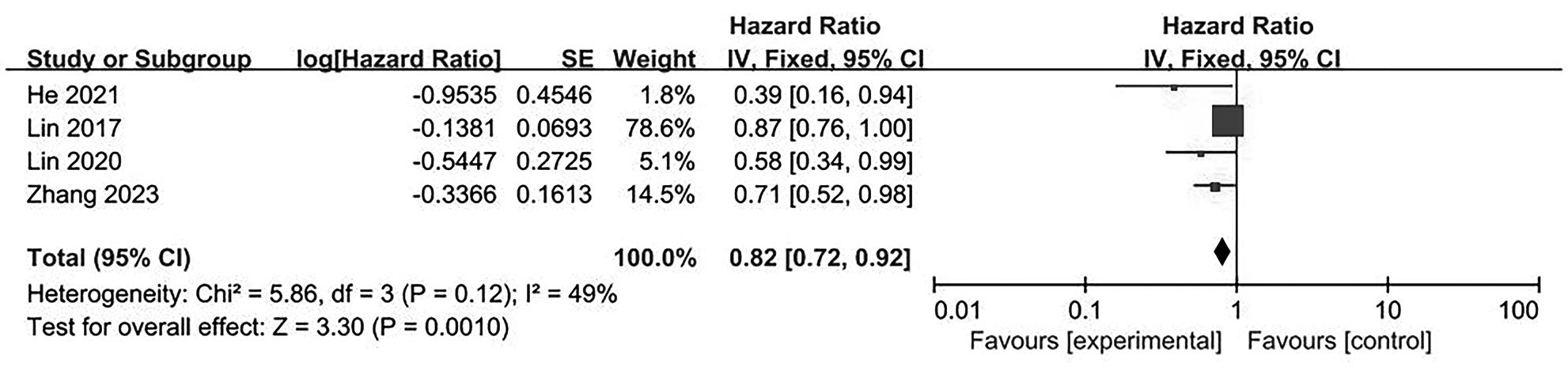
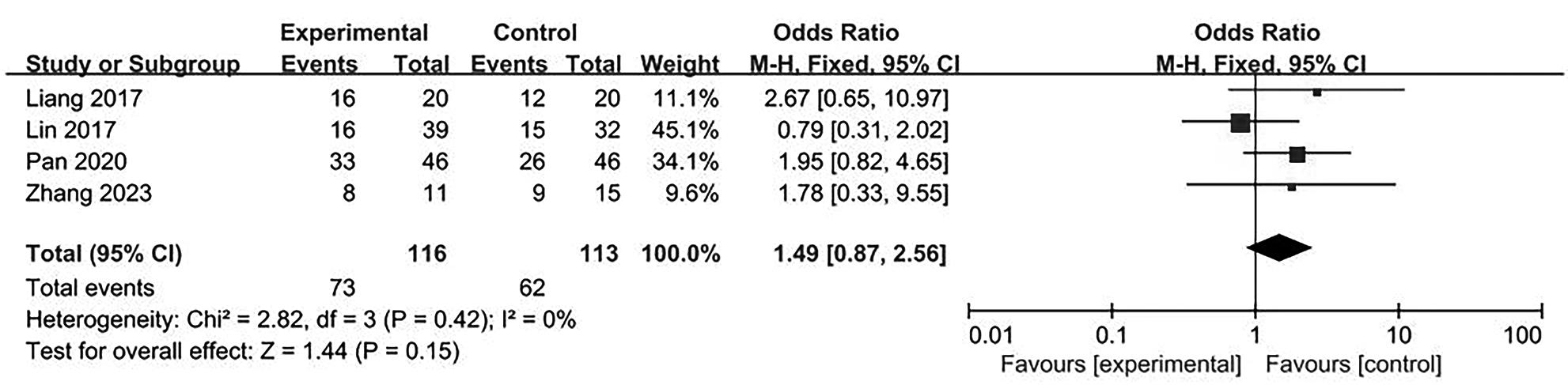
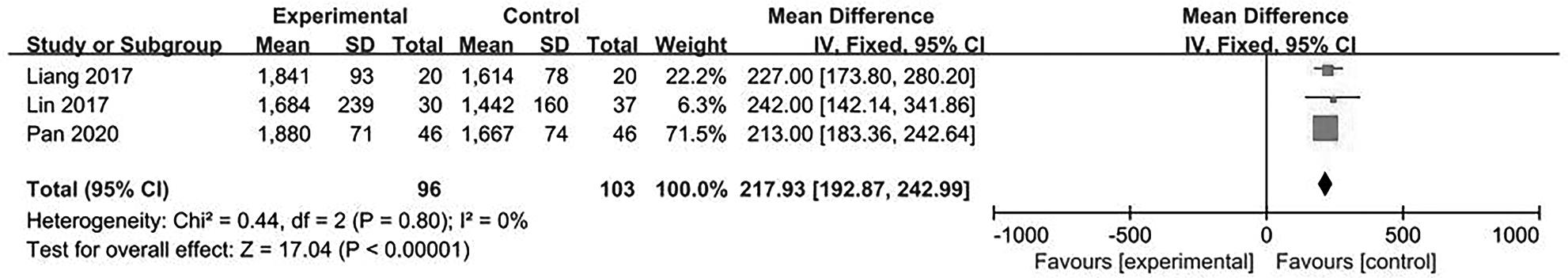
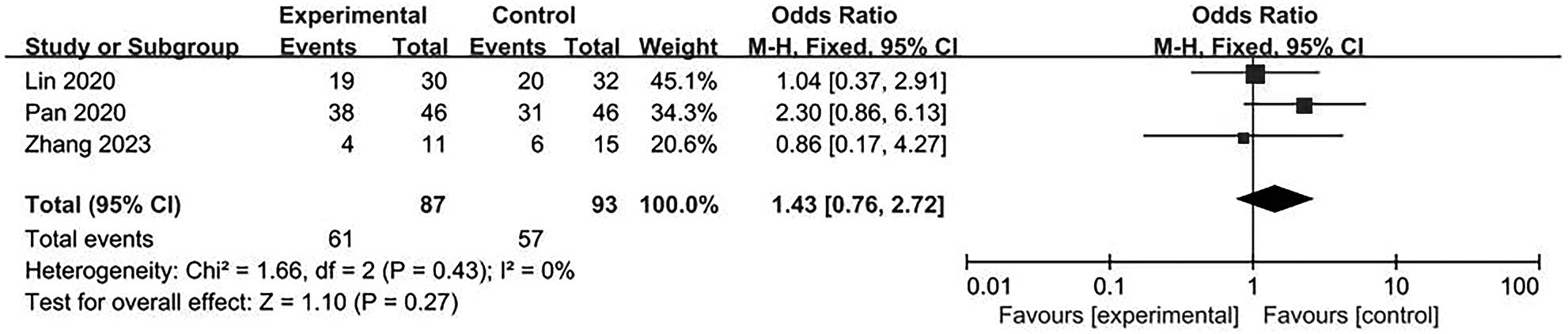
目的 系统评价不可逆电穿孔(IRE)联合免疫治疗对于不可切除胰腺癌患者的安全性和有效性。 方法 本研究根据PRISMA指南完成,PROSPERO注册号:CRD42024531984。检索PubMed、Embase、Cochrane Library、Web of Science、中国知网、万方和维普等数据库从建库至2024年2月发表的关于IRE联合免疫治疗不可切除胰腺癌的相关文献。按照设定的纳入和排除标准筛选文献并提取数据,评估文献质量。采用Review Manager 5.3和Stata 17.0软件进行Meta分析。 结果 最终纳入6篇研究,其中3篇前瞻性研究,2篇回顾性研究,1篇随机对照试验。共计376例不可切除胰腺癌患者,其中IRE治疗组222例,IRE联合免疫治疗组154例。Meta分析结果显示,IRE联合免疫治疗相较于IRE治疗组,能够明显提高患者的无进展生存期(HR=0.82,95%CI:0.72~0.92,P=0.001),延长总生存期(HR=0.86,95%CI:0.80~0.93,P=0.000 1),以及增加患者体内T淋巴细胞计数(MD=217.93,95%CI:192.87~242.99,P<0.000 01),提高患者机体免疫功能。但是在降低患者不良事件发生率(OR=1.43,95%CI:0.76~2.72,P=0.27)及提高患者疾病客观缓解率(OR=1.49,95%CI:0.87~2.56,P=0.15)方面差异均无统计学意义。 结论 IRE联合免疫治疗安全有效,可以明显提高不可切除胰腺癌患者总生存期与无进展生存期,增强患者免疫功能。 Abstract:Objective To systematically review the safety and efficacy of irreversible electroporation (IRE) combined with immunotherapy in patients with unresectable pancreatic cancer. Methods This study was conducted according to the PRISMA guideline, with a PROSPERO registration unmber of CRD42024531984. Datebases including PubMed, Embase the Cochrane Library, Web of Science, CNKI, Wanfang Data, and VIP were searched for related articles on IRE combined with immunotherapy for unresectable pancreatic cancer published up to February 2024. The articles were screened and related data were extracted according to the established inclusion and exclusion criteria, and the quality of the articles was assessed. Review Manager 5.3 and Stata 17.0 software were used to perform the meta-analysis. Results Six studies were finally included, with three prospective studies, two retrospective studies, and one randomized controlled trial. There were 376 patients with unresectable pancreatic cancer in total, among whom there were 222 patients in the IRE group and 154 patients in the IRE+immunotherapy group. The meta-analysis showed that compared with IRE alone, IRE combined with immunotherapy significantly prolonged progression-free survival (hazard ratio [HR]=0.82, 95% confidence interval [CI]: 0.72 — 0.92, P=0.001) and overall survival (HR=0.86, 95%CI: 0.80 — 0.93, P=0.000 1), increased T lymphocyte count in the patients (mean difference=217.93, 95%CI: 192.87 — 242.99, P<0.000 01), and improved the immune function of patients. However, there were no significant differences between the two groups in reducing the incidence rate of adverse events (odds ratio [OR]=1.43, 95%CI: 0.76 — 2.72, P=0.27) and improving the objective remission rate of patients (OR=1.49, 95%CI: 0.87 — 2.56, P=0.15). Conclusion IRE combined with immunotherapy is safe and effective in patients with unresectable pancreatic cancer and can significantly improve overall survival and progression-free survival and enhance immune function, with little effect on objective remission rate and the incidence rate of adverse events. -
Key words:
- Pancreatic Neoplasms /
- Ablation Techniquesl /
- Immunosuppression /
- Meta-Analysis
-
表 1 纳入研究的6篇文献基本特征
Table 1. Basic characteristics of the six included studies
作者 发表时间(年) 国家 例数 治疗方案 质量评分(分) 研究类型 Lin[12] 2017 中国 71 IRE+NK细胞 NOS评分:7 前瞻性研究 Lin[13] 2017 中国 40 IRE+NK细胞 NOS评分:7 前瞻性研究 Lin[14] 2020 中国 62 IRE+γδ2 T淋巴细胞 Jadad评分:4 随机对照试验 Pan[15] 2020 中国 92 IRE+NK细胞 NOS评分:8 前瞻性研究 He[16] 2021 中国 85 IRE+toripalimab NOS评分:7 回顾性研究 Zhang[17] 2023 中国 26 IRE+PD-1抑制剂 NOS评分:8 回顾性研究 -
[1] CAI J, CHEN HD, LU M, et al. Trend analysis on morbidity and mortality of pancreatic cancer in China, 2005-2015[J]. Chin J Epidemiol, 2021, 42( 5): 794- 800. DOI: 10.3760/cma.j.cn112338-20201115-01328.蔡洁, 陈宏达, 卢明, 等. 2005—2015年中国胰腺癌发病与死亡趋势分析[J]. 中华流行病学杂志, 2021, 42( 5): 794- 800. DOI: 10.3760/cma.j.cn112338-20201115-01328. [2] TEMPERO MA, MALAFA MP, AL-HAWARY M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 4): 439- 457. DOI: 10.6004/jnccn.2021.0017. [3] GEBOERS B, TIMMER FEF, RUARUS AH, et al. Irreversible electroporation and nivolumab combined with intratumoral administration of a toll-like receptor ligand, as a means of in vivo vaccination for metastatic pancreatic ductal adenocarcinoma(PANFIRE-III). A phase-I study protocol[J]. Cancers, 2021, 13( 15): 3902. DOI: 10.3390/cancers13153902. [4] TIMMER FEF, GEBOERS B, RUARUS AH, et al. Irreversible electroporation for locally advanced pancreatic cancer[J]. Tech Vasc Interv Radiol, 2020, 23( 2): 100675. DOI: 10.1016/j.tvir.2020.100675. [5] KWON W, THOMAS A, KLUGER MD. Irreversible electroporation of locally advanced pancreatic cancer[J]. Semin Oncol, 2021, 48( 1): 84- 94. DOI: 10.1053/j.seminoncol.2021.02.004. [6] DANG XD. Therapeutic effect analysis of irreversible electroporation for pancreatic cancer and preliminary exploration of combined therapy[D]. Yan’an: Yan’an University, 2023.党旭东. 不可逆电穿孔治疗胰腺癌疗效分析及联合治疗应用初步探索[D]. 延安: 延安大学, 2023. [7] TIMMER FEF, GEBOERS B, NIEUWENHUIZEN S, et al. Pancreatic cancer and immunotherapy: A clinical overview[J]. Cancers, 2021, 13( 16): 4138. DOI: 10.3390/cancers13164138. [8] HE CB, WANG J, SUN SX, et al. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer[J]. J Oncol, 2019, 2019: 9346017. DOI: 10.1155/2019/9346017. [9] PANDIT H, HONG YK, LI Y, et al. Evaluating the regulatory immunomodulation effect of irreversible electroporation(IRE) in pancreatic adenocarcinoma[J]. Ann Surg Oncol, 2019, 26( 3): 800- 806. DOI: 10.1245/s10434-018-07144-3. [10] SCHEFFER HJ, STAM AGM, GEBOERS B, et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation[J]. Oncoimmunology, 2019, 8( 11): 1652532. DOI: 10.1080/2162402X.2019.1652532. [11] WELLS G, SHEA BJ, O’CONNELL D, et al. The Newcastle-Ottawa Scale(NOS) for assessing the quality of nonrandomized studies in Meta-analyses[EB/OL].( 2021-05-03). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [12] LIN M, ALNAGGAR M, LIANG SZ, et al. An important discovery on combination of irreversible electroporation and allogeneic natural killer cell immunotherapy for unresectable pancreatic cancer[J]. Oncotarget, 2017, 8( 60): 101795- 101807. DOI: 10.18632/oncotarget.21974. [13] LIN M, LIANG SZ, WANG XH, et al. Short-term clinical efficacy of percutaneous irreversible electroporation combined with allogeneic natural killer cell for treating metastatic pancreatic cancer[J]. Immunol Lett, 2017, 186: 20- 27. DOI: 10.1016/j.imlet.2017.03.018. [14] LIN M, ZHANG XY, LIANG SZ, et al. Irreversible electroporation plus allogenic Vγ9Vδ2 T cells enhances antitumor effect for locally advanced pancreatic cancer patients[J]. Signal Transduct Target Ther, 2020, 5( 1): 215. DOI: 10.1038/s41392-020-00260-1. [15] PAN QH, HU CG, FAN YH, et al. Efficacy of irreversible electroporation ablation combined with natural killer cells in treating locally advanced pancreatic cancer[J]. J BUON, 2020, 25( 3): 1643- 1649. [16] HE CB, SUN SX, ZHANG Y, et al. Irreversible electroporation plus anti-PD-1 antibody versus irreversible electroporation alone for patients with locally advanced pancreatic cancer[J]. J Inflamm Res, 2021, 14: 4795- 4807. DOI: 10.2147/JIR.S331023. [17] ZHANG T, LI XY, CHEN YJ, et al. Clinical efficacy of IRE ablation combined with PD-1 inhibitor and chemotherapy in treatment of locally advanced pancreatic cancer[J]. J Pract Med, 2023, 39( 9): 1153- 1158. DOI: 10.3969/j.issn.1006-5725.2023.09.016.张通, 李晓勇, 陈艳军, 等. 不可逆电穿孔消融术联合程序性死亡蛋白-1抑制剂及化疗在局部进展期胰腺癌的临床疗效[J]. 实用医学杂志, 2023, 39( 9): 1153- 1158. DOI: 10.3969/j.issn.1006-5725.2023.09.016. [18] RUARUS AH, VROOMEN LGPH, PUIJK RS, et al. Irreversible electroporation in hepatopancreaticobiliary tumours[J]. Can Assoc Radiol J, 2018, 69( 1): 38- 50. DOI: 10.1016/j.carj.2017.10.005. [19] GUPTA P, MARALAKUNTE M, SAGAR S, et al. Efficacy and safety of irreversible electroporation for malignant liver tumors: A systematic review and meta-analysis[J]. Eur Radiol, 2021, 31( 9): 6511- 6521. DOI: 10.1007/s00330-021-07742-y. [20] ONG S, LEONARDO M, CHENGODU T, et al. Irreversible electroporation for prostate cancer[J]. Life(Basel), 2021, 11( 6): 490. DOI: 10.3390/life11060490. [21] FAIELLA E, SANTUCCI D, VERTULLI D, et al. Irreversible electroporation(IRE) for prostate cancer(PCa) treatment: The state of the art[J]. J Pers Med, 2024, 14( 2): 137. DOI: 10.3390/jpm14020137. [22] RINGEL-SCAIA VM, BEITEL-WHITE N, LORENZO MF, et al. High-frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti-tumor immunity[J]. EBioMedicine, 2019, 44: 112- 125. DOI: 10.1016/j.ebiom.2019.05.036. [23] KIZY S, BAEZ H, GABER O, et al. Immunomodulation after irreversible electroporation for locally advanced pancreatic cancer: A Meta-analysis and institutional series[J]. Annals of Surgical Oncology, 2022, 29( Suppl 2): S444- S445. [24] ERDEM S, NARAYANAN JS, WORNI M, et al. Local ablative therapies and the effect on antitumor immune responses in pancreatic cancer-A review[J]. Heliyon, 2024, 10( 1): e23551. DOI: 10.1016/j.heliyon.2023.e23551. [25] FEIG C, GOPINATHAN A, NEESSE A, et al. The pancreas cancer microenvironment[J]. Clin Cancer Res, 2012, 18( 16): 4266- 4276. DOI: 10.1158/1078-0432.CCR-11-3114. [26] THIND K, PADRNOS LJ, RAMANATHAN RK, et al. Immunotherapy in pancreatic cancer treatment: A new frontier[J]. Therap Adv Gastroenterol, 2017, 10( 1): 168- 194. DOI: 10.1177/1756283X16667909. [27] O’NEILL C, HAYAT T, HAMM J, et al. A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma[J]. Surgery, 2020, 168( 4): 610- 616. DOI: 10.1016/j.surg.2020.04.057. [28] BHUTIANI N, AGLE S, LI Y, et al. Irreversible electroporation enhances delivery of gemcitabine to pancreatic adenocarcinoma[J]. J Surg Oncol, 2016, 114( 2): 181- 186. DOI: 10.1002/jso.24288. [29] HE CB, HUANG X, ZHANG Y, et al. Comparison of survival between irreversible electroporation followed by chemotherapy and chemotherapy alone for locally advanced pancreatic cancer[J]. Front Oncol, 2020, 10: 6. DOI: 10.3389/fonc.2020.00006. [30] ZHAO BY, LIANG ZJ, ZHANG LX, et al. Safety and efficacy of irreversible electroporation combined with neoadjuvant chemotherapy for locally advanced pancreatic cancer: A meta-analysis[J]. Cancer Res Prev Treat, 2022, 49( 11): 1139- 1145. DOI: 10.3971/j.issn.1000-8578.2022.22.0367.赵宝银, 梁昭君, 张丽霞, 等. 不可逆电穿孔术联合新辅助化疗治疗局部进展期胰腺癌安全性和有效性的Meta分析[J]. 肿瘤防治研究, 2022, 49( 11): 1139- 1145. DOI: 10.3971/j.issn.1000-8578.2022.22.0367. [31] GEBOERS B, SCHEFFER HJ, GRAYBILL PM, et al. High-voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy[J]. Radiology, 2020, 295( 2): 254- 272. DOI: 10.1148/radiol.2020192190. [32] CHEN BT, MAO XH. Clinical research advance in immunotherapy of pancreatic cancer[J]. Chin J Dig Surg, 2023, 22( 5): 610- 615. DOI: 10.3760/cma.j.cn115610-20230407-00158.陈博滔, 毛先海. 胰腺癌免疫治疗临床研究进展[J]. 中华消化外科杂志, 2023, 22( 5): 610- 615. DOI: 10.3760/cma.j.cn115610-20230407-00158. [33] FINCHAM REA, DELVECCHIO FR, GOULART MR, et al. Natural killer cells in pancreatic cancer stroma[J]. World J Gastroenterol, 2021, 27( 24): 3483- 3501. DOI: 10.3748/wjg.v27.i24.3483. -



 PDF下载 ( 1024 KB)
PDF下载 ( 1024 KB)

 下载:
下载: