CXC型趋化因子配体10与12在胆囊癌组织中的表达水平及在肿瘤侵袭中的作用机制
DOI: 10.12449/JCH241120
Expression of CXCL10 and CXCL12 in gallbladder carcinoma and their mechanism of action in tumor invasion
-
摘要:
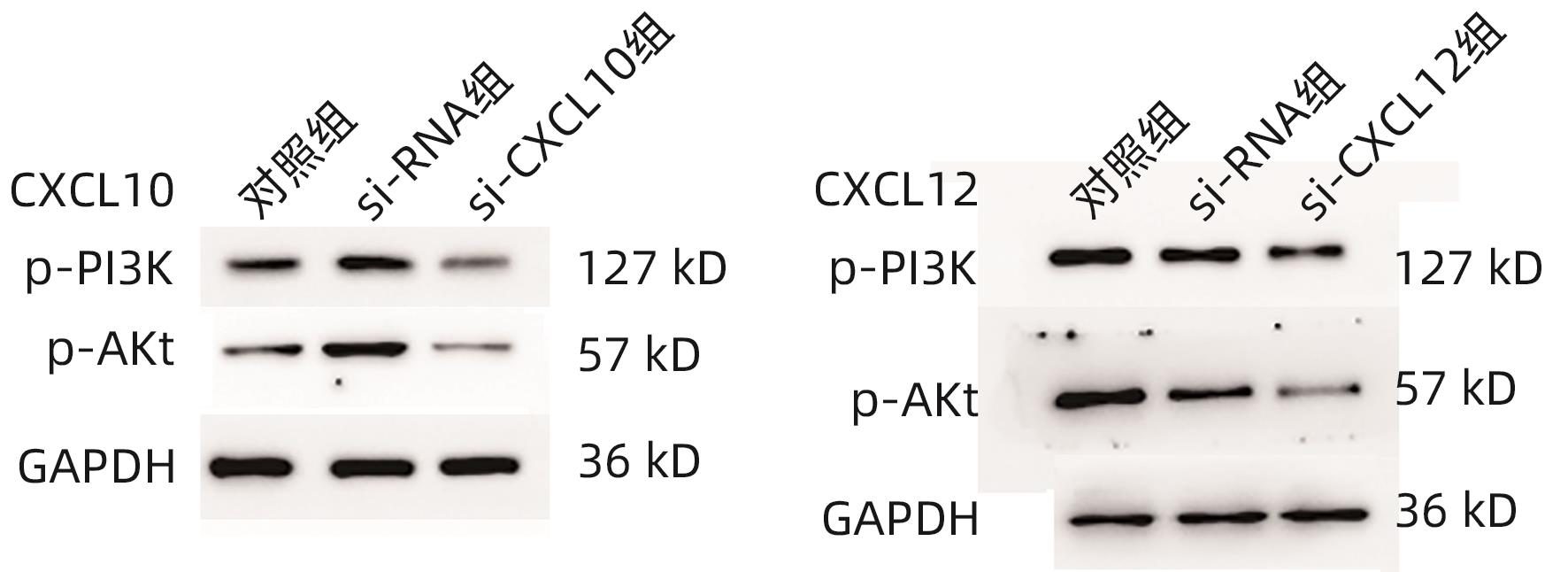
目的 探讨CXC型趋化因子配体10(CXCL10)和12在胆囊癌组织中的表达水平及其参与肿瘤侵袭的机制。 方法 选择2020年4月—2023年4月在中国人民解放军中部战区总医院手术切除的56例胆囊癌患者肿瘤组织及癌旁组织标本,使用RT-PCR法检测癌组织及癌旁组织CXCL10 mRNA、CXCL12 mRNA表达,分析患者癌组织中CXCL10 mRNA、CXCL12 mRNA表达与患者临床病理参数的关系。使用人胆囊癌细胞株GBC-SD,构建CXCL10、CXCL12低表达胆囊癌细胞,CCK-8法检测CXCL10、CXCL12低表达对胆囊癌细胞株增殖的影响,Transwell检测CXCL10、CXCL12低表达对胆囊癌细胞株侵袭能力的影响,并采用Western Blot法检测胆囊癌细胞PI3K/Akt通路表达情况。计量资料两组间比较采用配对t检验或成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。 结果 在胆囊癌患者中,癌组织CXCL10、CXCL12 mRNA相对表达量均显著高于癌旁组织(1.857±0.315 vs 1.024±0.203、2.038±0.374 vs 1.064±0.221,t值分别为16.342、16.778,P值均<0.05)。不同TNM分期、有无淋巴结转移、有无远处转移以及不同肿瘤直径患者的CXCL10、CXCL12 mRNA比较差异均有统计学意义(P值均<0.05)。CXCL10:si-CXCL10组细胞CXCL10 mRNA相对表达量、CXCL10蛋白表达、CCK-8吸光值、细胞迁移数量、p-PI3K及p-Akt蛋白表达均显著低于对照组和si-RNA组(P值均<0.05);CXCL12:si-CXCL12组细胞CXCL12 mRNA相对表达量、CXCL12蛋白表达、CCK-8吸光值、细胞迁移数量、p-PI3K及p-Akt蛋白表达均显著低于对照组和si-RNA组(P值均<0.05)。 结论 胆囊癌组织中的CXCL10及CXCL12表达升高,抑制CXCL10、CXCL12表达后,胆囊癌细胞增殖、侵袭受到明显抑制,其机制可能与抑制PI3K/Akt通路磷酸化有关。 -
关键词:
- 胆囊肿瘤 /
- 趋化因子CXCL10 /
- 趋化因子CXCL12 /
- 肿瘤浸润
Abstract:Objective To investigate the expression levels of CXCL10 and CXCL12 in gallbladder carcinoma and their mechanism of action in tumor invasion. Methods Tumor tissue samples and adjacent tissue samples were collected from 56 patients with gallbladder carcinoma who underwent surgical resection in General Hospital of Central Theater Command from April 2020 to April 2023. RT-PCR was used to measure the mRNA expression levels of CXCL10 and CXCL12 in cancerous tissue and adjacent tissue, and the correlation of the mRNA expression levels of CXCL10 and CXCL12 in cancerous tissue with clinicopathological parameters was analyzed. The human gallbladder carcinoma cell line GBC-SD was used to construct gallbladder carcinoma cells with low expression of CXCL10 and CXCL12. CCK8 assay was used to observe the effect of low expression of CXCL10 and CXCL12 on the proliferation of gallbladder carcinoma cells, Transwell assay was used to observe the effect of low expression of CXCL10 and CXCL12 on the invasion ability of gallbladder carcinoma cells, and Western blot was used to measure the expression of the PI3K/Akt pathway in gallbladder carcinoma cells. The paired t-test or independent-samples t-test was used for comparison of measurement data between two groups; an analysis of variance was used for comparison between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results In the patients with gallbladder carcinoma, the relative mRNA expression levels of CXCL10 and CXCL12 in cancerous tissue were significantly higher than those in adjacent tissue (CXCL10: 1.857±0.315 vs 1.024±0.203, t=16.342, P<0.05; CXCL12: 2.038±0.374 vs 1.064±0.221, t=16.778, P<0.05). There were significant differences in the relative mRNA expression levels of CXCL10 and CXCL12 between the patients with different TNM stages, presence or absence of lymph node metastasis or distant metastasis, and tumor diameters (all P<0.05). Compared with the control group and the si-RNA group, the si-CXCL10 group had significantly lower relative mRNA and protein expression levels of CXCL10 and CXCL12, CCK-8 absorbance values, number of cell migration, and protein expression levels of p-PI3K and p-Akt (all P<0.05). Conclusion There are increases in the expression of CXCL10 and CXCL12 in gallbladder carcinoma tissue, and the proliferation and invasion of gallbladder carcinoma cells are significantly inhibited after inhibition of the expression of CXCL10 and CXCL12, which might be associated with the inhibition of the phosphorylation of the PI3K/Akt pathway. -
Key words:
- Gallbladder Neoplasms /
- Chemokine CXCL10 /
- Chemokine CXCL12 /
- Neoplasm Invasiveness
-
表 1 癌组织及癌旁组织CXCL10 mRNA、CXCL12 mRNA表达比较
Table 1. Comparison of CXCL10 mRNA and CXCL12 mRNA expression in cancer tissue and adjacent tissue
组别 例数 CXCL10 mRNA CXCL12 mRNA 癌组织 56 1.857±0.315 2.038±0.374 癌旁组织 56 1.024±0.203 1.064±0.221 t值 18.359 20.506 P值 <0.001 <0.001 表 2 癌组织CXCL10 mRNA、CXCL12 mRNA表达与患者临床病理参数的关系
Table 2. The relationship between the expression of CXCL10 mRNA and CXCL12 mRNA in cancer tissues and clinical pathological parameters of patients
临床病理特征 例数 CXCL10 mRNA CXCL12 mRNA 性别 男 27 1.796±0.293 1.985±0.337 女 29 1.914±0.356 2.083±0.405 t值 1.359 0.980 P值 0.183 0.331 年龄 ≥60岁 25 1.894±0.229 1.993±0.311 <60岁 31 1.827±0.336 2.074±0.401 t值 0.850 0.828 P值 0.399 0.411 TNM分期 Ⅰ~Ⅱ期 21 1.447±0.285 1.570±0.324 Ⅲ~Ⅳ期 35 2.103±0.407 2.319±0.452 t值 6.483 6.630 P值 <0.001 <0.001 病理类型 腺癌 49 1.844±0.394 2.015±0.416 其他 7 1.948±0.410 2.199±0.375 t值 0.650 1.106 P值 0.518 0.274 淋巴结转移 有 30 2.153±0.473 2.349±0.332 无 26 1.515±0.338 1.679±0.415 t值 5.724 6.709 P值 <0.001 <0.001 远处转移 有 19 2.203±0.374 2.412±0.423 无 37 1.679±0.310 1.846±0.325 t值 5.580 5.561 P值 <0.001 <0.001 肿瘤直径 >5 cm 21 2.115±0.263 2.414±0.462 ≤5 cm 35 1.702±0.364 1.812±0.394 t值 4.531 5.187 P值 <0.001 <0.001 表 3 各组细胞CXCL10、CXCL12 mRNA及蛋白的比较
Table 3. Comparison of CXCL10 and CXCL12 mRNA and protein expression among different groups
分组 mRNA 蛋白 CXCL10 对照组 1.029±0.204 0.937±0.152 si-RNA组 1.048±0.1561) 1.035±0.2241) si-CXCL10组 0.492±0.1151)2) 0.533±0.1281)2) F值 18.878 4.453 P值 <0.001 0.036 CXCL12 对照组 0.937±0.152 1.685±0.217 si-RNA组 1.035±0.2241) 1.603±0.3011) si-CXCL12组 0.533±0.1281)2) 0.997±0.2051)2) F值 11.845 9.186 P值 <0.001 <0.001 注:与对照组比较,1)P<0.05;与si-RNA组比较,2)P<0.05。
表 4 各组细胞吸光值和迁移数量的比较
Table 4. Comparison of light absorption value and migration number of cells in each group
分组 吸光值 细胞迁移数量 CXCL10 对照组 1.44±0.32 159.43±24.09 si-RNA组 1.39±0.401) 150.35±31.541) si-CXCL10组 0.74±0.151)2) 108.30±25.601)2) F值 8.029 5.005 P值 0.006 0.026 CXCL12 对照组 1.51±0.23 162.39±39.42 si-RNA组 1.59±0.331) 158.23±41.391) si-CXCL12组 0.86±0.201)2) 101.31±29.841)2) F值 11.915 4.202 P值 0.001 0.041 注:与对照组比较,1)P<0.05;与si-RNA组比较,2)P<0.05。
表 5 各组细胞PI3K/Akt通路蛋白的比较
Table 5. Comparison of PI3K/Akt pathway proteins in each group
分组 p-PI3K p-Akt CXCL10 对照组 0.984±0.132 1.294±0.120 si-RNA组 1.023±0.1741) 1.317±0.2191) si-CXCL10组 0.485±0.0971)2) 0.720±0.1371)2) F值 23.638 21.152 P值 0.006 <0.001 CXCL12 对照组 1.127±0.133 1.423±0.210 si-RNA组 1.094±0.1651) 1.389±0.1971) si-CXCL12组 0.603±0.1021)2) 0.792±0.1741)2) F值 23.353 16.692 P值 <0.001 <0.001 注:与对照组比较,1)P<0.05;与si-RNA组比较,2)P<0.05。
-
[1] ROA JC, BASTURK O, ADSAY V. Dysplasia and carcinoma of the gallbladder: Pathological evaluation, sampling, differential diagnosis and clinical implications[J]. Histopathology, 2021, 79( 1): 2- 19. DOI: 10.1111/his.14360. [2] GIRALDO NA, DRILL E, SATRAVADA BA, et al. Comprehensive molecular characterization of gallbladder carcinoma and potential targets for intervention[J]. Clin Cancer Res, 2022, 28( 24): 5359- 5367. DOI: 10.1158/1078-0432.CCR-22-1954. [3] LIU ZX, ZHOU SB, ZHANG ZY, et al. SDC1 knockdown promotes invasion and migration of gallbladder cancer cells via ERK signaling pathway[J]. J Mod Oncol, 2021, 29( 21): 3726- 3731. DOI: 10.3969/j.issn.1672-4992.2021.21.006.刘子祥, 周少波, 张子艳, 等. 沉默SDC1通过ERK信号通路促进胆囊癌细胞的侵袭和迁移[J]. 现代肿瘤医学, 2021, 29( 21): 3726- 3731. DOI: 10.3969/j.issn.1672-4992.2021.21.006. [4] KORBECKI J, KOJDER K, KAPCZUK P, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors-a review of literature[J]. Int J Mol Sci, 2021, 22( 2): 843. DOI: 10.3390/ijms22020843. [5] SUN XT, HE XK, ZHANG Y, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism[J]. Gut, 2022, 71( 1): 129- 147. DOI: 10.1136/gutjnl-2020-322744. [6] Biliary Surgery Group of Surgery Branch of Chinese Medical Association. Guideline for the diagnosis and treatment of gallbladder carcinoma(2015 edition)[J]. J Clin Hepatol, 2016, 32( 3): 411- 419. DOI: 10.3969/j.issn.1001-5256.2016.03.002.中华医学会外科学分会胆道外科学组. 胆囊癌诊断和治疗指南(2015版)[J]. 临床肝胆病杂志, 2016, 32( 3): 411- 419. DOI: 10.3969/j.issn.1001-5256.2016.03.002. [7] YUAN B, ZHAO XF, WANG X, et al. Patient-derived organoids for personalized gallbladder cancer modelling and drug screening[J]. Clin Transl Med, 2022, 12( 1): e678. DOI: 10.1002/ctm2.678. [8] LIU YB, CHEN W. Current situation and prospect in the clinical treatment of gallbladder cancer[J]. Chin J Dig Surg, 2023, 22( 1): 81- 88. DOI: 10.3760/cma.j.cn115610-20230109-00013.刘颖斌, 陈炜. 胆囊癌临床治疗的现状与展望[J]. 中华消化外科杂志, 2023, 22( 1): 81- 88. DOI: 10.3760/cma.j.cn115610-20230109-00013. [9] KAMAYA A, FUNG C, SZPAKOWSKI JL, et al. Management of incidentally detected gallbladder polyps: Society of radiologists in ultrasound consensus conference recommendations[J]. Radiology, 2022, 305( 2): 277- 289. DOI: 10.1148/radiol.213079. [10] WANG LM, XU MF, KAO CY, et al. Small molecule JQ1 promotes prostate cancer invasion via BET-independent inactivation of FOXA1[J]. J Clin Invest, 2020, 130( 4): 1782- 1792. DOI: 10.1172/JCI126327. [11] WAN GQ, LIU YH, ZHU J, et al. SLFN5 suppresses cancer cell migration and invasion by inhibiting MT1-MMP expression via AKT/GSK-3β/β-catenin pathway[J]. Cell Signal, 2019, 59: 1- 12. DOI: 10.1016/j.cellsig.2019.03.004. [12] LEI JJ, ZHANG J, ZHANG D, et al. Research progress of perineural invasion in gallbladder cancer[J]. Chin J Dig Surg, 2023, 22( 7): 933- 937. DOI: 10.3760/cma.j.cn115610-20230606-00266.雷建军, 张健, 张东, 等. 胆囊癌神经浸润研究进展[J]. 中华消化外科杂志, 2023, 22( 7): 933- 937. DOI: 10.3760/cma.j.cn115610-20230606-00266. [13] ZHANG GL, LUO X, ZHANG W, et al. CXCL-13 regulates resistance to 5-fluorouracil in colorectal cancer[J]. Cancer Res Treat, 2020, 52( 2): 622- 633. DOI: 10.4143/crt.2019.593. [14] YANG YR, LI JY, LEI WR, et al. CXCL12-CXCR4/CXCR7 axis in cancer: From mechanisms to clinical applications[J]. Int J Biol Sci, 2023, 19( 11): 3341- 3359. DOI: 10.7150/ijbs.82317. [15] HEIDEGGER I, FOTAKIS G, OFFERMANN A, et al. Comprehensive characterization of the prostate tumor microenvironment identifies CXCR4/CXCL12 crosstalk as a novel antiangiogenic therapeutic target in prostate cancer[J]. Mol Cancer, 2022, 21( 1): 132. DOI: 10.1186/s12943-022-01597-7. [16] ZHANG YY, LI J, NIU YX, et al. Expression of CXC chemokine ligand 13 in cervical cancer and its effect on proliferation and migration of cervical cancer cells[J]. Chin J Gerontol, 2023, 43( 10): 2534- 2537. DOI: 10.3969/j.issn.1005-9202.2023.10.062.张燕怡, 李娟, 牛煜欣, 等. 宫颈癌中CXC趋化因子配体13表达及其对宫颈癌细胞增殖和迁移的影响[J]. 中国老年学杂志, 2023, 43( 10): 2534- 2537. DOI: 10.3969/j.issn.1005-9202.2023.10.062. [17] ZHOU Y, SHEN XN, CHEN ZH, et al. Expression and clinical significance of CC chemokine receptor 4 in colorectal cancer[J]. China Med Herald, 2023, 20( 11): 19- 22, 36. DOI: 10.20047/j.issn1673-7210.2023.11.04.周元, 沈徐宁, 陈治横, 等. 结直肠癌中CC趋化因子受体4的表达及临床意义[J]. 中国医药导报, 2023, 20( 11): 19- 22, 36. DOI: 10.20047/j.issn1673-7210.2023.11.04. [18] CHANG XD, LI HY, CHEN J, et al. The role and mechanism of human umbilical cord-derived mesenchymal stem cells in invasive of human pancreatic cancer cells[J]. Clin J Med Off, 2022, 50( 12): 1211- 1214. DOI: 10.16680/j.1671-3826.2022.12.01.常旭东, 李宏宇, 陈江, 等. 人脐带间充质干细胞对人胰腺癌细胞侵袭能力调控作用及机制研究[J]. 临床军医杂志, 2022, 50( 12): 1211- 1214. DOI: 10.16680/j.1671-3826.2022.12.01. [19] CHEN DL, SHENG H, ZHANG DS, et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p[J]. Mol Cancer, 2021, 20( 1): 166. DOI: 10.1186/s12943-021-01475-8. [20] D’ALTERIO C, GIARDINO A, SCOGNAMIGLIO G, et al. CXCR4-CXCL12-CXCR7 and PD-1/PD-L1 in pancreatic cancer: CXCL12 predicts survival of radically resected patients[J]. Cells, 2022, 11( 21): 3340. DOI: 10.3390/cells11213340. [21] HIRTH M, GANDLA J, HÖPER C, et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients[J]. Gastroenterology, 2020, 159( 2): 665- 681. e 13. DOI: 10.1053/j.gastro.2020.04.037. [22] GLAVIANO A, FOO ASC, LAM HY, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer[J]. Mol Cancer, 2023, 22( 1): 138. DOI: 10.1186/s12943-023-01827-6. [23] EDIRIWEERA MK, TENNEKOON KH, SAMARAKOON SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance[J]. Semin Cancer Biol, 2019, 59: 147- 160. DOI: 10.1016/j.semcancer.2019.05.012. -



 PDF下载 ( 2736 KB)
PDF下载 ( 2736 KB)

 下载:
下载: