白细胞介素22对肝星状细胞活化的影响及其机制
DOI: 10.12449/JCH241116
-
摘要:
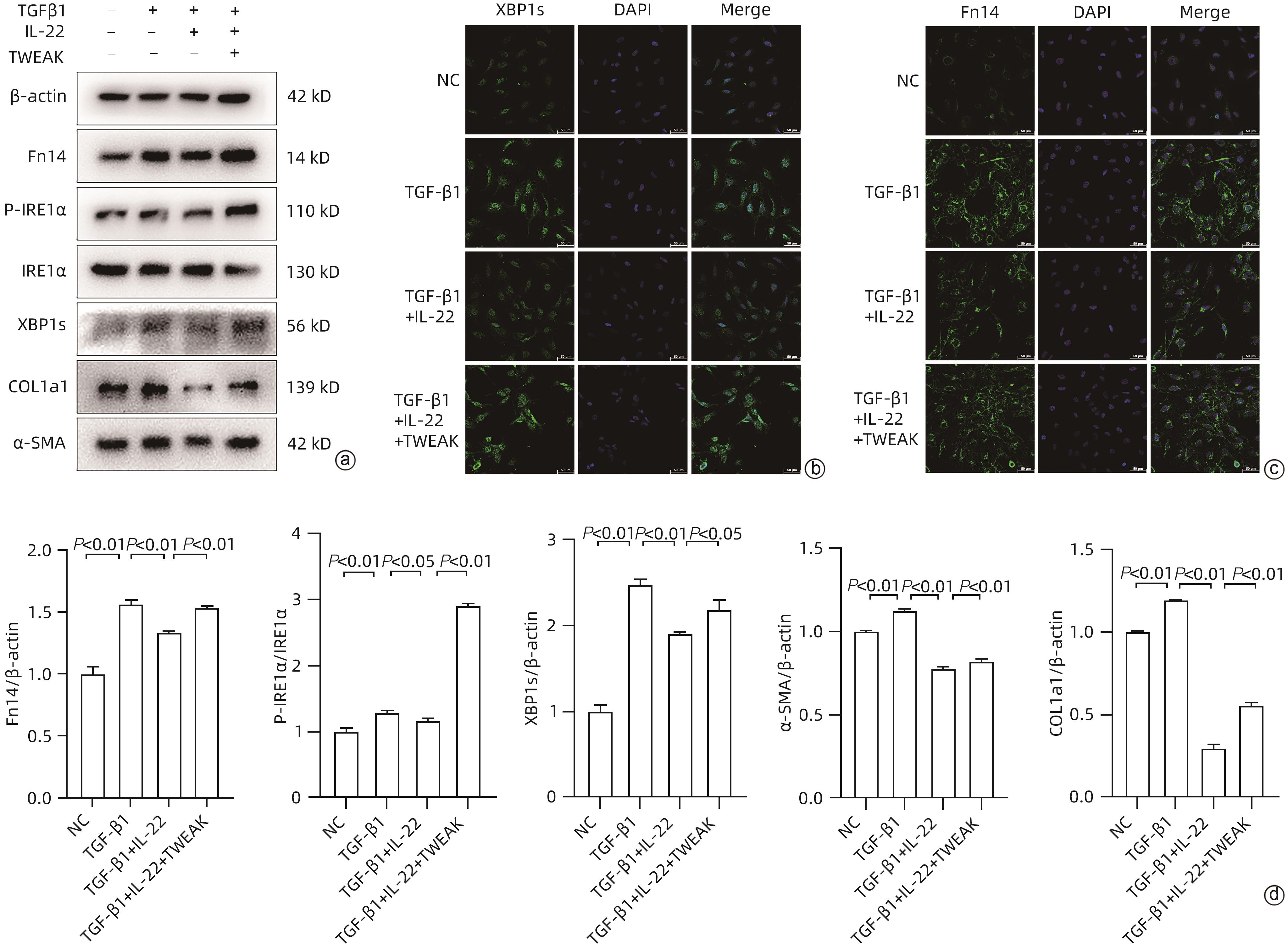
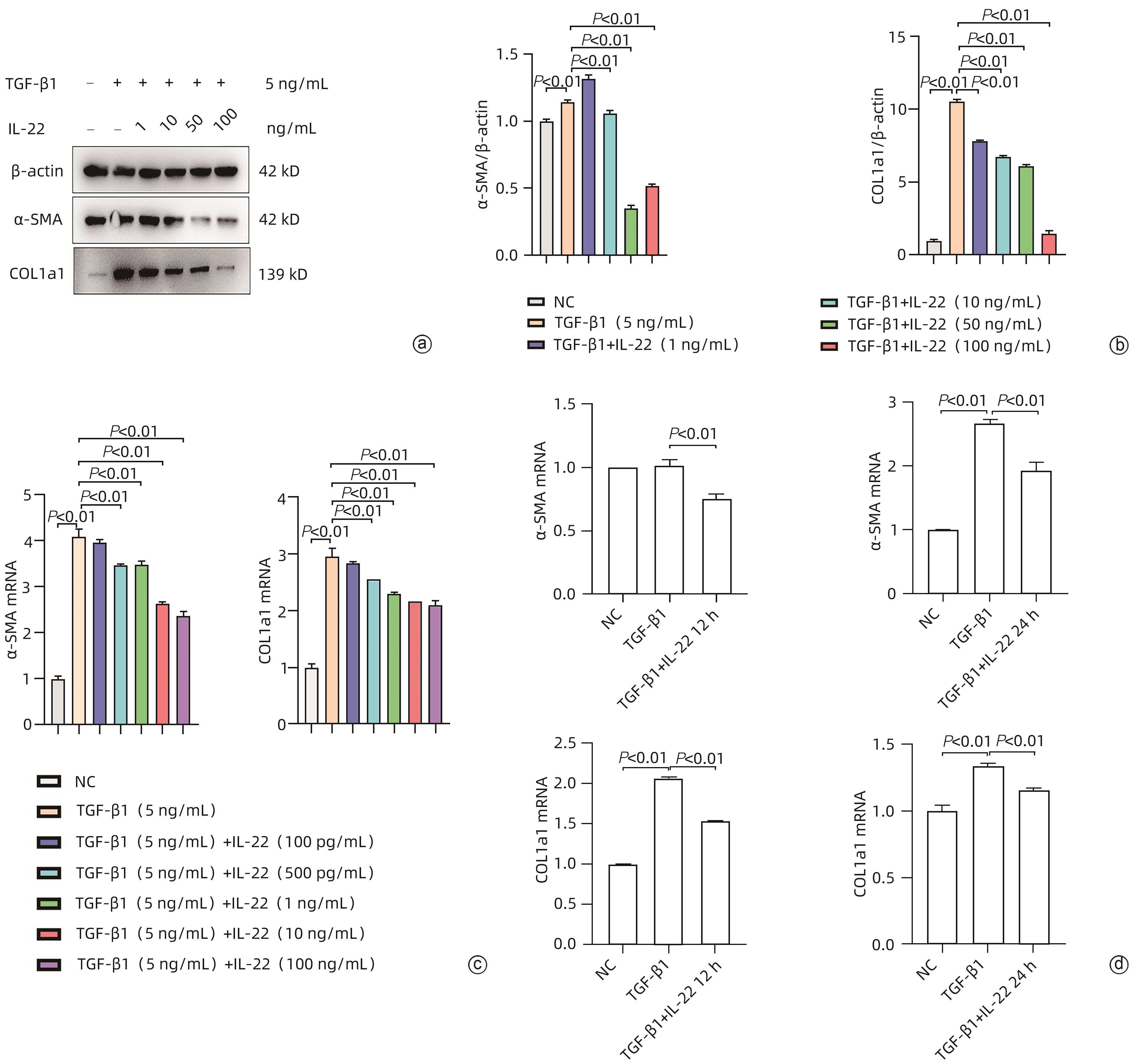
目的 探讨肝星状细胞活化过程中白细胞介素(IL)22发挥的作用及影响机制。 方法 选取人肝星状细胞系LX-2细胞为研究对象,以转化生长因子(TGF)β1诱导LX-2细胞构建肝星状细胞活化模型,以梯度浓度的IL-22处理LX-2细胞,通过Western Blot、qRT-PCR检测活化标志物Ⅰ型胶原蛋白(COL1A1)、α-平滑肌肌动蛋白(α-SMA)表达水平以确定适宜的药物工作浓度、时间;通过Western Blot、qRT-PCR及免疫荧光方法检测经IL-22处理的活化肝星状细胞中成纤维细胞因子诱导早期反应蛋白14(Fn14)、内质网应激(ERS)及其活化标志物水平;以衣霉素(TM)诱导LX-2细胞ERS,通过Western Blot、qRT-PCR检测经IL-22处理后LX-2细胞ERS及其活化标志物水平;使用肿瘤坏死因子样细胞凋亡弱诱导剂(TWEAK)、小干扰RNA分别上/下调Fn14,再检测磷酸化肌醇需求蛋白1α(p-IRE1α)、肌醇需求蛋白1α(IRE1α)、转录因子剪接型X-盒结合蛋白1(XBP1s)、COL1A1和α-SMA基因及蛋白水平;在IL-22处理TGF-β1诱导的LX-2细胞的基础上加用TWEAK上调Fn14,通过Western Blot、免疫荧光方法检测Fn14、ERS及其活化标志物水平。计量资料两组间比较采用成组t检验;多组间比较采用单因素方差分析,进一步两两比较采用Sidak’s多重比较检验。 结果 与TGF-β1组相比,TGFβ1+IL-22组COL1A1、α-SMA的蛋白和mRNA表达水平均下降,且在IL-22浓度为10 ng/mL以上作用24小时时效果更加显著(P值均<0.01);与TGF-β1组相比,TGF-β1+IL-22组Fn14、p-IRE1α、XBP1s表达水平均下降(P值均<0.05);与TM组相比,TM+IL-22组p-IRE1α、XBP1s、COL1A1和α-SMA表达水平均下降(P值均<0.05);与沉默对照(NC)组相比,Fn14 siRNA组p-IRE1α、XBP1s、COL1A1和α-SMA表达水平均下降(P值均<0.05);与正常对照组相比,TWEAK组Fn14、p-IRE1α、XBP1s、COL1A1和α-SMA表达水平均上升(P值均<0.01);与TGF-β1+IL-22组相比,TGF-β1+IL-22+TWEAK组Fn14、p-IRE1α、XBP1s、COL1A1和α-SMA表达水平均上升(P值均<0.05)。 结论 IL-22通过抑制Fn14负调控肝星状细胞ERS进而抑制其活化增殖。 Abstract:Objective To investigate the effect of interleukin-22 (IL-22) on the activation of hepatic stellate cells (HSCs) and its mechanism. Methods The human HSC LX-2 cells were selected for the study, and the LX-2 cells induced by TGF-β1 were used to establish a model of HSC activation. LX-2 cells were treated with IL-22 at gradient concentrations, and Western blot and qRT-PCR were used to measure the expression levels of the activation markers COL1A1 and α-SMA and determine the appropriate working concentration and time of the drug. Western blot, qRT-PCR, and immunofluorescence assay were used to determine the levels of Fn14 and the markers for endoplasmic reticulum stress (ERS) and activation in activated HSCs treated by IL-22. ERS in LX-2 cells was induced by tunicamycin (TM), and Western blot and qRT-PCR were used to measure the levels of markers for ERS and activation in LX-2 cells treated by IL-22. TNF-like weak inducer of apoptosis (TWEAK) and small interfering RNA were used to upregulate and downregulate Fn14, and then the mRNA and protein expression levels of p-IRE1α, IRE1α, XBP1s, COL1A1, and α-SMA were measured. After LX-2 cells induced by TGF-β1 were treated by IL-22, TWEAK was used to upregulate Fn14, and Western blot and immunofluorescence assay were used to measure the levels of Fn14 and the markers for ERS and activation. The independent-samples t-test was used for comparison of continuous data between two groups; a one-way analysis of variance was used for comparison between multiple groups, and the Sidak’s multiple comparison test was used for further comparison between two groups. Results Compared with the TGF-β1 group, the TGF-β1+IL-22 group had significant reductions in the protein and mRNA expression levels of COL1A1 and α-SMA, with a more significant effect after treatment with 10 ng/mL IL-22 for 24 hours (all P<0.01). Compared with the TGF-β1 group, the TGF-β1+IL-22 group had significant reductions in the expression levels of Fn14, p-IRE1α, and XBP1s (all P<0.05). Compared with the TM group, the TM+IL-22 group had significant reductions in the expression levels of p-IRE1α, XBP1s, COL1A1, and α-SMA (all P<0.05). Compared with the silenced control group, the Fn14 siRNA group had significant reductions in the expression levels of p-IRE1α, XBP1s, COL1A1, and α-SMA (all P<0.05). Compared with the normal control group, the TWEAK group had significant increases in the expression levels of Fn14, p-IRE1α, XBP1s, COL1A1, and α-SMA (all P<0.01). Compared with the TGFβ1+IL-22 group, the TGF-β1+IL-22+TWEAK group had significant increases in the expression levels of Fn14, p-IRE1α, XBP1s, COL1A1, and α-SMA (all P<0.05). Conclusion IL-22 negatively regulates ERS in HSCs by inhibiting Fn14, thereby inhibiting the activation of HSCs. -
Key words:
- Hepatic Fibrosis /
- Interleukin-22 /
- Hepatic Stellate Cells /
- Endoplasmic Reticulum Stress
-
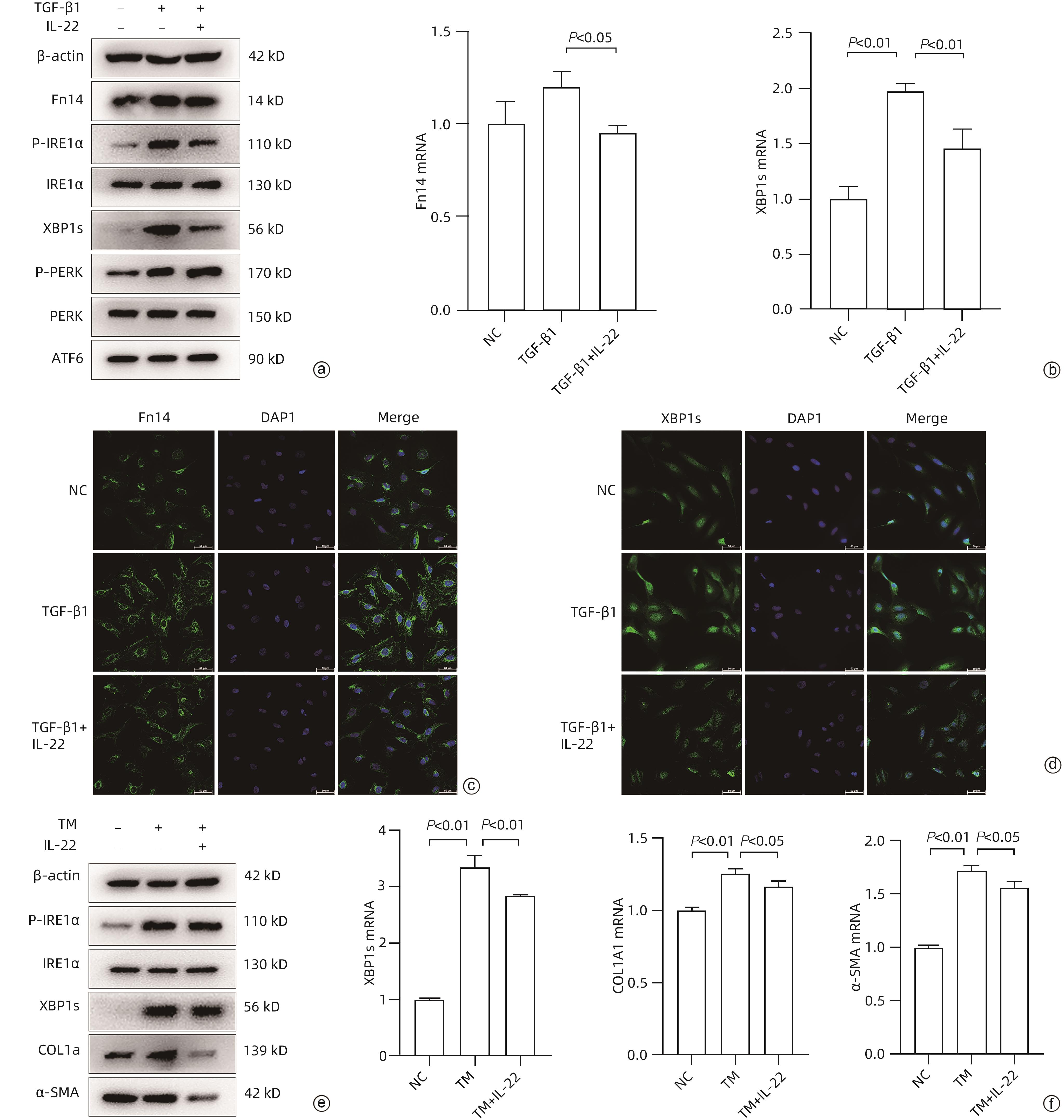
注: a,Western Blot分析LX-2细胞经不同分组(NC、TGF-β1、TGF-β1+IL-22)处理24 h后,相关目的蛋白表达情况;b,qRT-PCR分析LX-2细胞经同a图方法处理后XBP1s、Fn14基因表达量;c、d为免疫荧光分析LX-2细胞同a图方法处理后Fn14、XBP1s表达量(×400);e,Western Blot分析LX-2细胞经不同分组(NC、TM、TM+IL-22)处理24 h后相关目的蛋白表达情况;f,qRT-PCR分析LX-2细胞经同e图方法处理后XBP1s、COL1A1、α-SMA基因表达量。
图 2 IL-22抑制肝星状细胞中Fn14的表达和ERS
Figure 2. IL-22 inhibits Fn14 expression and ERS in HSCs
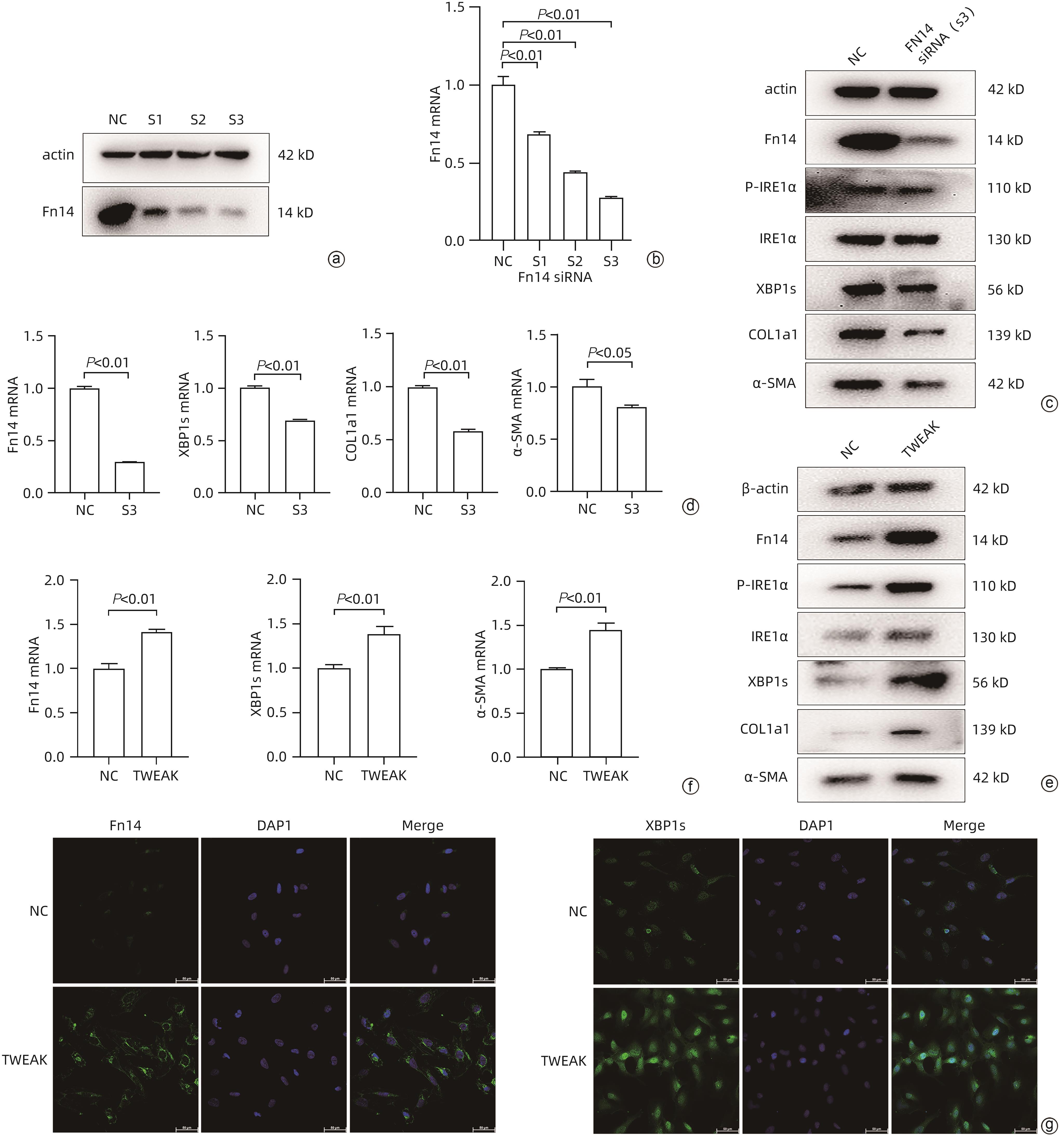
注: a、b,Western Blot、qRT-PCR分析不同Fn14 siRNA的沉默效果,筛选沉默效率最佳的S3;c,Western Blot分析沉默Fn14表达对LX-2细胞相关蛋白表达影响;d,qRT-PCR分析沉默Fn14表达对LX-2细胞XBP1s、COL1A1、α-SMA基因表达影响;e,Western Blot分析TWEAK蛋白处理对LX-2细胞相关蛋白表达影响;f,qRT-PCR分析TWEAK蛋白处理对LX-2细胞Fn14、XBP1s、α-SMA基因表达影响;g,免疫荧光分析TWEAK蛋白处理对LX-2细胞XBP1s、Fn14表达量影响(×400)。
图 3 Fn14调控IRE1α-XBP1s信号通路
Figure 3. Fn14 regulates the IRE1α-XBP1s signaling pathway
表 1 目标基因的引物序列
Table 1. Primer sequences of target genes
基因 正向引物(5'-3') 反向引物(5'-3') β-actin GGCACCACACCTTCTACAATGAG GGATAGCACAGCCTGGATAGCA α-SMA CCTGTGTTGTGGTTTACACTGG GGGGGAATTATCTTTCCTGGTCC COL1A1 GAGGGCCAAGACGAAGACATC CAGATCACGTCATCGCACAAC XBP1s TGGATTCTGGCGGTATTGACTC GAACTGGGTCCTTCTGGGTAGA Fn14 GCTCTGAGCCTGACCTTCGT TCTCTCCTGCGGCATCGT -
[1] GINÈS P, KRAG A, ABRALDES JG, et al. Liver cirrhosis[J]. Lancet, 2021, 398( 10308): 1359- 1376. DOI: 10.1016/s0140-6736(21)01374-x. [2] DEWIDAR B, MEYER C, DOOLEY S, et al. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019[J]. Cells, 2019, 8( 11): 1419. DOI: 10.3390/cells8111419. [3] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 3): 151- 166. DOI: 10.1038/s41575-020-00372-7. [4] CROFT M, SIEGEL RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases[J]. Nat Rev Rheumatol, 2017, 13( 4): 217- 233. DOI: 10.1038/nrrheum.2017.22. [5] WANG M, XIE ZJ, XU J, et al. TWEAK/Fn14 axis in respiratory diseases[J]. Clin Chim Acta, 2020, 509: 139- 148. DOI: 10.1016/j.cca.2020.06.007. [6] XU MC, ZHANG F, WANG AX, et al. Tumor necrosis factor-like weak inducer of apoptosis promotes hepatic stellate cells migration via canonical NF-κB/MMP9 pathway[J]. PLoS One, 2016, 11( 12): e0167658. DOI: 10.1371/journal.pone.0167658. [7] WANG AX, ZHANG F, XU H, et al. TWEAK/Fn14 promotes pro-inflammatory cytokine secretion in hepatic stellate cells via NF-κB/STAT3 pathways[J]. Mol Immunol, 2017, 87: 67- 75. DOI: 10.1016/j.molimm.2017.04.003. [8] LIU YM, VERMA VK, MALHI H, et al. Lipopolysaccharide downregulates macrophage-derived IL-22 to modulate alcohol-induced hepatocyte cell death[J]. Am J Physiol Cell Physiol, 2017, 313( 3): C305- C313. DOI: 10.1152/ajpcell.00005.2017. [9] KONG XN, FENG DC, WANG H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice[J]. Hepatology, 2012, 56( 3): 1150- 1159. DOI: 10.1002/hep.25744. [10] LU DH, GUO XY, QIN SY, et al. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines[J]. World J Gastroenterol, 2015, 21( 5): 1531- 1545. DOI: 10.3748/wjg.v21.i5.1531. [11] ZHAO JJ, ZHANG Z, LUAN Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment[J]. Hepatology, 2014, 59( 4): 1331- 1342. DOI: 10.1002/hep.26916. [12] WU LY, LIU SH, LIU Y, et al. Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C[J]. Clin Immunol, 2015, 158( 1): 77- 87. DOI: 10.1016/j.clim.2015.03.003. [13] YAP KN, YAMADA K, ZIKELI S, et al. Evaluating endoplasmic reticulum stress and unfolded protein response through the lens of ecology and evolution[J]. Biol Rev Camb Philos Soc, 2021, 96( 2): 541- 556. DOI: 10.1111/brv.12667. [14] JALAN R, CHIARA FD, BALASUBRAMANIYAN V, et al. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension[J]. J Hepatol, 2016, 64( 4): 823- 833. DOI: 10.1016/j.jhep.2015.11.019. [15] ZHU J, WANG RW, XU T, et al. Salvianolic acid A attenuates endoplasmic reticulum stress and protects against cholestasis-induced liver fibrosis via the SIRT1/HSF1 pathway[J]. Front Pharmacol, 2018, 9: 1277. DOI: 10.3389/fphar.2018.01277. [16] KIM RS, HASEGAWA D, GOOSSENS N, et al. The XBP1 arm of the unfolded protein response induces fibrogenic activity in hepatic stellate cells through autophagy[J]. Sci Rep, 2016, 6: 39342. DOI: 10.1038/srep39342. [17] HU ML, YANG SL, YANG L, et al. Interleukin-22 alleviated palmitate-induced endoplasmic reticulum stress in INS-1 cells through activation of autophagy[J]. PLoS One, 2016, 11( 1): e0146818. DOI: 10.1371/journal.pone.0146818. [18] GULHANE M, MURRAY L, LOURIE R, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22[J]. Sci Rep, 2016, 6: 28990. DOI: 10.1038/srep28990. [19] ROEHLEN N, CROUCHET E, BAUMERT TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives[J]. Cells, 2020, 9( 4): 875. DOI: 10.3390/cells9040875. [20] ZHOU X, WANG Z, HE XR, et al. Research advances in signaling pathways associated with potential anti-liver fibrosis drugs and targets[J]. J Clin Hepatol, 2023, 39( 12): 2932- 2941. DOI: 10.3969/j.issn.1001-5256.2023.12.027.周鑫, 王智, 何雪茹, 等. 潜在抗肝纤维化药物与靶点相关信号通路研究进展[J]. 临床肝胆病杂志, 2023, 39( 12): 2932- 2941. DOI: 10.3969/j.issn.1001-5256.2023.12.027. [21] WU Y, MIN J, GE C, et al. Interleukin 22 in liver injury, inflammation and cancer[J]. Int J Biol Sci, 2020, 16( 13): 2405- 2413. DOI: 10.7150/ijbs.38925. [22] EL-SHORBAGY AA, SHAFAA MW, SALAH ELBELTAGY R, et al. Liposomal IL-22 ameliorates liver fibrosis through miR-let7a/STAT3 signaling in mice[J]. Int Immunopharmacol, 2023, 124( Pt B): 111015. DOI: 10.1016/j.intimp.2023.111015. [23] MENG YX, HUO LJ. Role of interleukin-22 in the development and progression of liver fibrosis[J]. J Clin Hepatol, 2021, 37( 12): 2924- 2927. DOI: 10.3969/j.issn.1001-5256.2021.12.039.孟昱希, 霍丽娟. IL-22在肝纤维化发生发展中的作用[J]. 临床肝胆病杂志, 2021, 37( 12): 2924- 2927. DOI: 10.3969/j.issn.1001-5256.2021.12.039. [24] GUPTA RK, FUNG K, FIGUEROA DS, et al. Integrative keratinocyte responses to TWEAK with IL-13 and IL-22 reveal pathogenic transcriptomes associated with atopic dermatitis[J]. J Invest Dermatol, 2024, 144( 5): 1071- 1074. DOI: 10.1016/j.jid.2023.11.009. [25] NA M, YANG XB, DENG YK, et al. Endoplasmic reticulum stress in the pathogenesis of alcoholic liver disease[J]. PeerJ, 2023, 11: e16398. DOI: 10.7717/peerj.16398. [26] NAGAR P, SHARMA P, DHAPOLA R, et al. Endoplasmic reticulum stress in Alzheimer’s disease: Molecular mechanisms and therapeutic prospects[J]. Life Sci, 2023, 330: 121983. DOI: 10.1016/j.lfs.2023.121983. [27] ZARAFSHANI M, MAHMOODZADEH H, SOLEIMANI V, et al. Expression and clinical significance of IRE1-XBP1s, p62, and caspase-3 in colorectal cancer patients[J]. Iran J Med Sci, 2024, 49( 1): 10- 21. DOI: 10.30476/IJMS.2023.96922.2856. [28] LIU L, ZHAO ML, JIN X, et al. Adaptive endoplasmic reticulum stress signalling via IRE1α-XBP1 preserves self-renewal of haematopoietic and pre-leukaemic stem cells[J]. Nat Cell Biol, 2019, 21( 3): 328- 337. DOI: 10.1038/s41556-019-0285-6. [29] MAIERS JL, KOSTALLARI E, MUSHREF M, et al. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice[J]. Hepatology, 2017, 65( 3): 983- 998. DOI: 10.1002/hep.28921. [30] ABDELFATTAH AM, MAHMOUD SS, EL-WAFAEY DI, et al. Diacerein ameliorates cholestasis-induced liver fibrosis in rat via modulating HMGB1/RAGE/NF-κB/JNK pathway and endoplasmic reticulum stress[J]. Sci Rep, 2023, 13( 1): 11455. DOI: 10.1038/s41598-023-38375-4. -



 PDF下载 ( 4369 KB)
PDF下载 ( 4369 KB)

 下载:
下载: