下瘀血汤对非酒精性脂肪性肝病小鼠模型肾损伤的干预作用及其机制
DOI: 10.12449/JCH241114
Effect of Xiayuxue Decoction against renal injury in mice with non-alcoholic fatty liver disease and its mechanism
-
摘要:
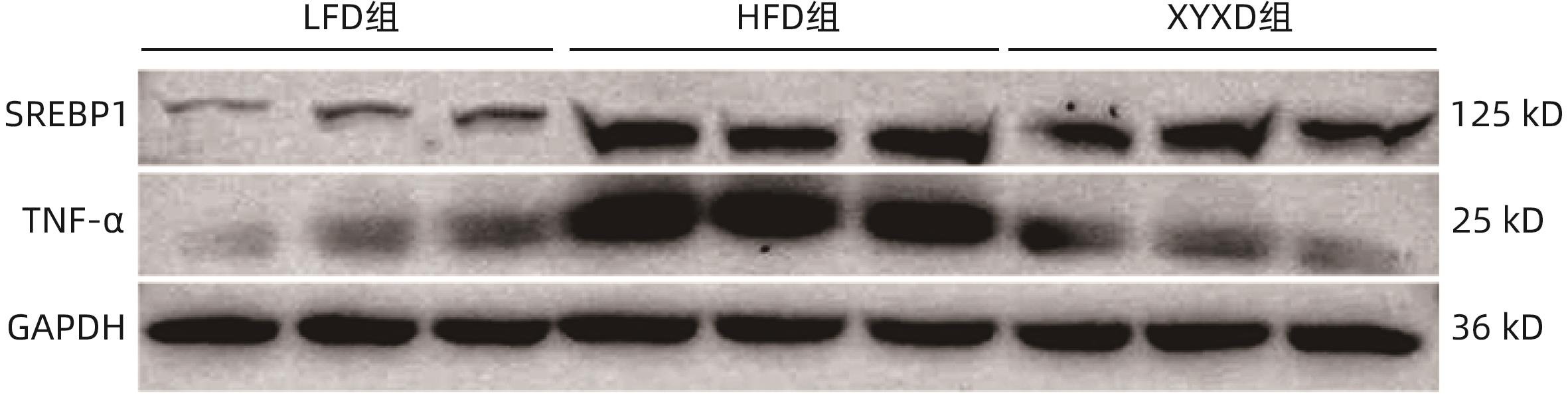
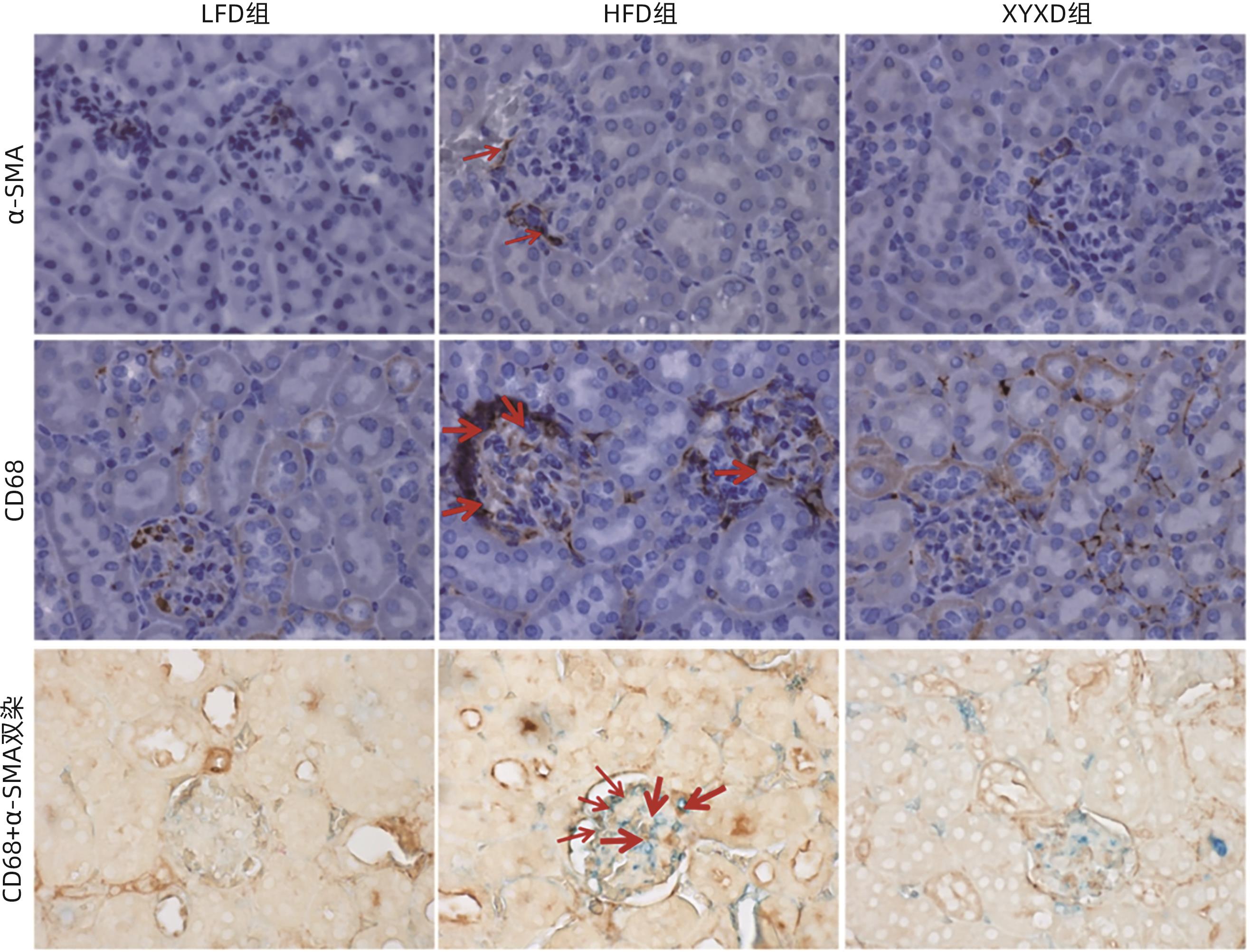
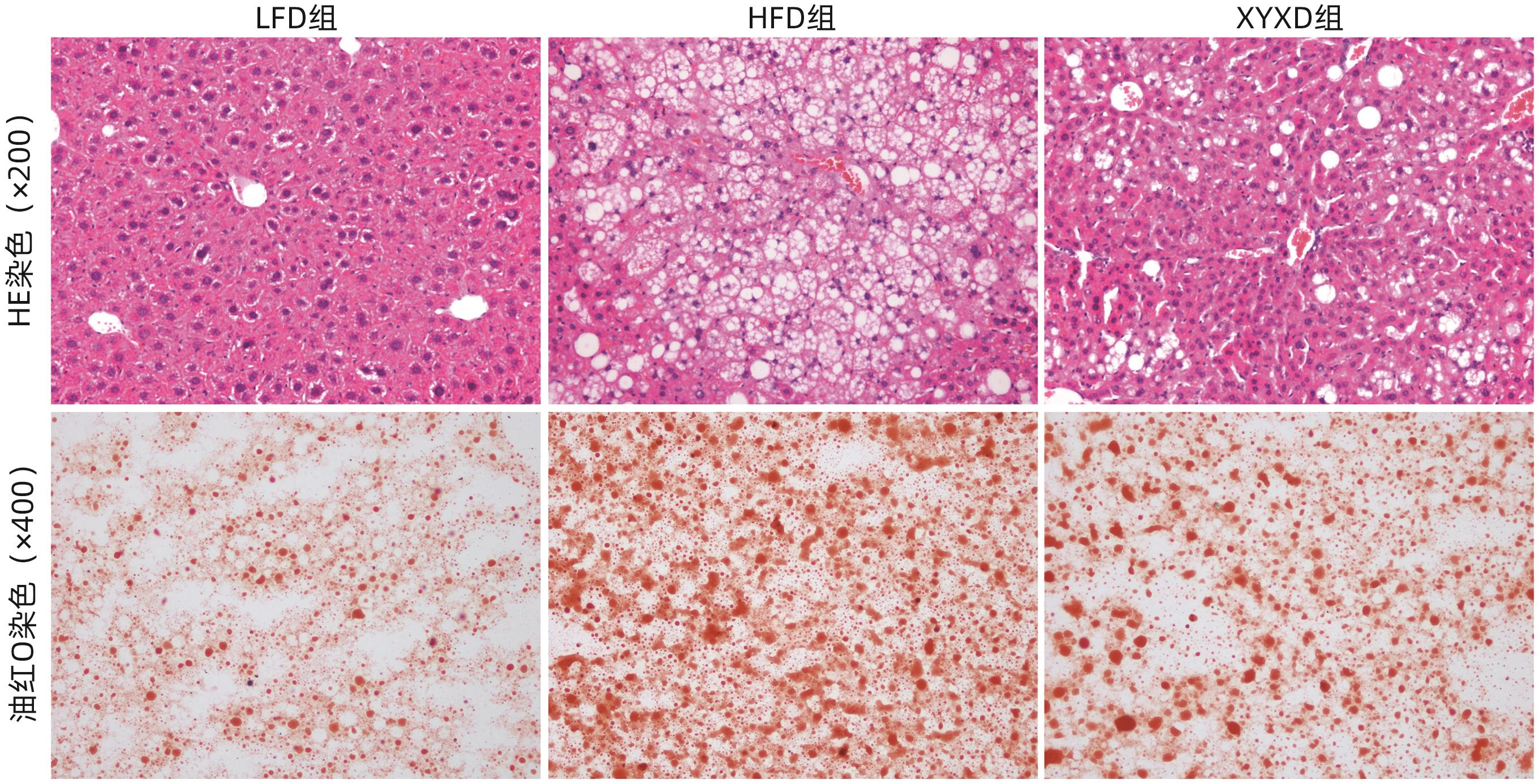
目的 探讨高脂饮食(HFD)诱导非酒精性脂肪性肝病(NAFLD)对小鼠肾脏的影响,以及下瘀血汤对NAFLD小鼠模型的保护作用及其机制。 方法 选取2020年9月—2021年9月在上海中医药大学附属普陀医院健康对照及NAFLD患者各25例,检测总胆固醇(TC)、甘油三酯(TG)、尿素氮(BUN)、肌酐(Cr)及尿酸(UA)水平;将24只雄性C57BL/6小鼠随机分为对照组(LFD组)、高脂组(HFD组)及高脂+下瘀血汤组(XYXD组),每组8只,第13周开始给予下瘀血汤每日1次灌胃至18周末,维持6周。检测各组TC、TG、BUN及Cr水平。HE、油红O染色观察肝脏和肾脏病理变化,免疫组化双染技术检测CD68和α-SMA表达。实时荧光定量PCR检测肾组织固醇调节元件结合蛋白1(SREBP1)、脂肪酸合酶(FASN)、IL-6、TNF-α、结蛋白(Desmin)和α-平滑肌肌动蛋白(α-SMA)的mRNA表达。Western Blot检测SREBP1和TNF-α蛋白水平。计量资料多组间比较采用单因素方差分析,两两比较采用LSD-t检验;两组间比较采用成组t检验。 结果 与健康对照组患者相比,NAFLD患者TC、TG、BUN、Cr、UA水平均升高(P值均<0.05)。与LFD组小鼠相比,HFD组小鼠体质量、TC、TG、BUN、Cr水平均升高(P值均<0.001);与HFD组相比,XYXD显著抑制NAFLD小鼠TC、TG、BUN、CR表达(P值均<0.001)。肝脏病理学显示:HFD组小鼠肝脂肪变及炎症浸润较LFD组显著增加,而XYXD组病变显著减轻。肾脏病理学显示:与LFD组相比,HFD组肾组织炎性浸润明显,脂肪变显著,肾脏胶原形成明显;与HFD组相比,XYXD显著减轻炎症浸润、抑制脂肪变及肾脏胶原形成。实时荧光定量PCR结果显示:与LFD组相比,HFD组肾组织中SREBP1、FASN、IL-6、TNF-α、Desmin和α-SMA的mRNA相对表达水平均显著升高(P值均<0.001),XYXD组较HFD组均显著降低(P值均<0.001)。Western Blot结果显示:与LFD组比较,HFD组SREBP1及TNF-α蛋白表达水平均明显上调(P值均<0.05);与HFD组比较,XYXD组SREBP1及TNF-α蛋白表达水平均明显下调(P值均<0.05)。免疫组化染色结果显示:HFD组α-SMA和CD68阳性染色及双阳性染色较LFD组均显著升高(P值均<0.05),XYXD组较HFD组均显著下降(P值均<0.05)。 结论 HFD可诱导肾脏脂肪变、炎症浸润及胶原形成,下瘀血汤可能通过抑制肾组织巨噬细胞与肌成纤维细胞表达发挥肾脏保护作用。 -
关键词:
- 非酒精性脂肪性肝病 /
- 膳食, 高脂 /
- 肾损伤 /
- 下瘀血汤 /
- 小鼠, 近交C57BL
Abstract:Objective To investigate the effect of non-alcoholic fatty liver disease (NAFLD) induced by high-fat diet (HFD) on the kidneys of mice and the protective effect and mechanism of Xiayuxue Decoction. Methods A total of 25 healthy controls and 25 NAFLD patients who attended Putuo Hospital Affiliated to Shanghai University of Traditional Chinese Medicine from September 2020 to September 2021 were enrolled, and the levels of total cholesterol (TC), triglyceride (TG), blood urea nitrogen (BUN), creatinine (Cr), and uric acid (UA) were measured. A total of 24 male C57BL/6 mice were randomly divided into low-fat diet (LFD) group, HFD group, and HFD+Xiayuxue Decoction group (XYXD group), with 8 mice in each group, and since week 13, XYXD was administered by gavage once a day for 6 weeks till the end of week 18. The level of TC, TG, BUN, and Cr were measured for each group. HE staining and oil red staining were used to observe the pathological changes of the liver and the kidneys; immunohistochemical double staining was used to measure the expression levels of CD68 and alpha-smooth muscle actin (α-SMA); quantitative real-time PCR was used to measure the expression levels of sterol regulatory element binding protein 1 (SREBP1), fatty acid synthase (FASN), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), Desmin, and α-SMA in renal tissue; Western blot was used to measure the protein expression levels of SREBP1 and TNF-α. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for pairwise comparison; the independent-samples t-test was used for comparison between two groups. Results Compared with the healthy controls, NAFLD patients showed significant increases in the levels of TC, TG, BUN, Cr, and UA (all P<0.05). Compared with the LFD group, the HFD group had significant increases in body weight, TC, TG, BUN, and Cr (all P<0.001), and compared with the HFD group, the XYXD group showed significant inhibition of the expression of TC, TG, BUN, and Cr (all P<0.001). Liver pathological examination showed that compared with the LFD group, the HFD group showed significant increases in hepatic steatosis and inflammatory infiltration, while the XYXD group showed significant alleviation of lesions. Renal pathological examination showed that compared with the LFD group, the HFD group had significant inflammatory infiltration, steatosis, and collagen formation in renal tissue, and compared with the HFD group, XYXD significantly alleviated inflammatory infiltration and inhibited steatosis and collagen formation. Quantitative real-time PCR showed that compared with the LFD group, the HFD group had significant increases in the relative mRNA expression levels of SREBP1, FASN, IL-6, TNF-α, Desmin, and α-SMA in renal tissue (all P<0.001), and compared with the HFD group, the XYXD group had significant reductions in the relative expression levels of these indicators (all P<0.001). Western blot showed that compared with the LFD group, the HFD group had significant increases in the protein expression levels of SREBP1 and TNF-α (P<0.05), and compared with the HFD group, the XYXD group had significant reductions in the protein expression levels of SREBP1 and TNF-α (P<0.05). Immunohistochemical staining showed that compared with the LFD group, the HFD group had significant increases in the positive staining or the double positive staining of α- SMA and CD68 (P<0.05), and compared with the HFD group, the XYXD group showed significant reductions (P<0.05). Conclusion HFD can induce renal steatosis, inflammatory infiltration, and collagen formation, and XYXD might exert a protective effect on the kidneys by inhibiting the expression of macrophages and myofibroblasts in renal tissue. -
表 1 引物序列
Table 1. Primer sequence
名称 序列 18S F:5'-GTAACCCGTTGAACCCCATT-3'
R:5'-CCATCCAATCGGTAGTAGCG-3'
TNF-α F:5'-ATGAGAGGGAGGCCATTTG-3'
R:5'-CAGCCTCTTCTCATTCCTGC-3'
IL-6 F:5'-ACCAGAGGAAATTTTCAATAGGC-3'
R:5'-TGATGCACTTGCAGAAAACA-3'
FASN F:5'-GGAGGTGGTGATAGCCGGTAT-3'
R:5'-TGGGTAATCCATAGAGCCCAG-3'
SREBP -1c F:5'-GCAGCCACCATCTAGCCTG-3' R 5'-CAGCAGTGAGTCTGCCTTGAT-3' α-SMA F 5'-GTTCAGTGGTGCCTCTGTCA-3' R 5'-ACTGGGACGACATGGAAAAG-3' Desmin F 5'-TCGGCTCTAAGGGCTCCTC-3' R 5'-CGTGGTCAGAAACTCCTGGTT-3' 表 2 研究对象血脂与肾功能参数的分析
Table 2. Analysis of blood lipids and renal function parameters in subjects
组别 例数 年龄(岁) BUN(mmol/L) Cr(μmol/L) UA(μmol/L) TC(mmol/L) TG(mmol/L) 对照组 25 41.88±12.12 4.33±1.02 53.52±3.06 265.00±9.43 4.14±0.78 1.15±0.53 NAFLD组 25 48.38±13.82 4.49±1.04 79.33±3.06 381.00±18.87 6.21±1.21 3.01±1.32 t值 1.73 2.05 5.97 5.50 15.00 13.23 P值 0.090 0.012 <0.001 <0.001 <0.001 <0.001 表 3 各组小鼠体质量肾功能、血脂表达改变
Table 3. Changes in body weight, renal function, and lipid expression of mice in each group
组别 动物数(只) 体质量(g) BUN(mmol/L) Cr(μmol/L) TC(mmol/L) TG(mmol/L) LFD组 8 30.74±2.34 6.14±0.38 5.29±0.30 2.63±0.23 0.53±0.03 HFD组 8 46.34±2.271) 7.49±0.121) 8.63±0.221) 5.46±0.141) 0.83±0.031) XYXD组 8 37.98±2.842) 6.64±0.252) 7.53±0.142) 3.62±0.192) 0.71±0.022) F值 78.13 75.70 432.41 474.30 312.04 P值 <0.001 <0.001 <0.001 <0.001 <0.001 注:与LFD组比较,1)P<0.001;与HFD组比较,2)P<0.001。
表 4 各组小鼠肾脏病理学、炎症纤维化指标变化
Table 4. Changes of pathological in each group
指标 LFD组 HFD组 XYXD组 F值 P值 NAS评分(分) 0.65±0.05 6.04±0.461) 3.61±0.192) 8.74 <0.001 天狼星红染色阳性面积百分比(%) 2.20±0.34 5.45±0.171) 3.26±0.122) 23.73 <0.001 油红O染色阳性面积百分比(%) 7.44±0.87 21.05±0.931) 14.88±0.152) 1.33 <0.001 免疫印迹 TNF-α 1.00±0.22 11.06±2.691) 8.49±2.672) 16.67 <0.001 SREBP-1 1.00±0.03 2.36±0.281) 1.16±0.272) 28.59 <0.001 免疫组化染色阳性面积百分比(%) CD68 0.25±0.06 1.77±0.201) 0.92±0.082) 4.94 <0.001 α-SMA 0.48±0.09 2.29±0.151) 1.49±0.152) 2.71 <0.001 CD68+α-SMA双染色 1.69±0.27 4.79±0.501) 3.40±0.492) 64.54 <0.001 RT-PCR TNF-α 1.00±0.21 2.14±0.491) 1.32±0.192) 26.12 <0.001 IL-6 1.00±0.27 2.34±0.971) 1.21±0.122) 12.92 <0.001 FASN 1.00±0.21 10.05±1.131) 7.30±0.842) 35.42 <0.001 SREBP-1c 1.00±0.35 11.76±1.251) 9.06±1.032) 25.82 <0.001 Desmin 1.00±0.18 2.15±0.871) 1.11±0.192) 11.86 <0.001 α-SMA 1.00±0.57 3.76±0.731) 1.70±0.262) 53.53 <0.001 注:与 LFD组比较,1)P<0.001;与 HFD 组比较:2)P<0.001。
-
[1] TARGHER G, BYRNE CD, TILG H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications[J]. Gut, 2024, 73( 4): 691- 702. DOI: 10.1136/gutjnl-2023-330595. [2] ESLAM M, AHMED A, DESPRÉS JP, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases[J]. Lancet Gastroenterol Hepatol, 2021, 6( 9): 743- 753. DOI: 10.1016/S2468-1253(21)00132-1. [3] PAIK JM, HENRY L, YOUNOSSI ZM. Nonalcoholic fatty liver disease mortality may not be decreasing: A need for careful interpretation of GBD 2019 estimates of liver deaths[J]. Cell Metab, 2023, 35( 7): 1087- 1088. DOI: 10.1016/j.cmet.2023.06.012. [4] MANTOVANI A, PETRACCA G, BEATRICE G, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: An updated meta-analysis[J]. Gut, 2022, 71( 1): 156- 162. DOI: 10.1136/gutjnl-2020-323082. [5] DAI KM. Jiang Chunhua’s experience in applying Xiayuxue Decoction[J]. Shanxi J Tradit Chin Med, 2012, 28( 1): 4- 6. DOI: 10.3969/j.issn.1000-7156.2012.01.002.戴克敏. 姜春华运用下瘀血汤的经验[J]. 山西中医, 2012, 28( 1): 4- 6. DOI: 10.3969/j.issn.1000-7156.2012.01.002. [6] SHEN DX, MA WT, WU L, et al. Mechanism of Xiayuxue Decoction on improving liver fibrosis by inhibiting pancreatic macrophage infiltration[J]. Acad J Shanghai Univ Tradit Chin Med, 2019, 33( 2): 66- 72, 79. DOI: 10.16306/j.1008-861x.2019.02.015.沈东晓, 马文婷, 吴柳, 等. 下瘀血汤抑制胰腺巨噬细胞浸润改善肝纤维化的机制研究[J]. 上海中医药大学学报, 2019, 33( 2): 66- 72, 79. DOI: 10.16306/j.1008-861x.2019.02.015. [7] LIU C, CAI J, CHENG Z, et al. Xiayuxue decoction reduces renal injury by promoting macrophage apoptosis in hepatic cirrhotic rats[J]. Genet Mol Res, 2015, 14( 3): 10760- 10773. DOI: 10.4238/2015.September.9.15. [8] DING SD, CHEN BC, LIU Y, et al. Proteomic study of Xiayuxue Decoction on liver cirrhosis of rats[J]. Chin Tradit Herb Drugs, 2012, 43( 1): 131- 138.丁赛丹, 陈必成, 刘艳, 等. 下瘀血汤干预肝硬化大鼠的蛋白质组学研究[J]. 中草药, 2012, 43( 1): 131- 138. [9] WU L, ZHANG J, MA WT, et al. Xiayuxue decoction inhibits methionine-choline-deficient-induced nonalcoholic steatohepatitis in mice[J/CD]. Chin J Liver Dis Electron Version, 2018, 10( 3): 48- 55. DOI: 10.3969/j.issn.1674-7380.2018.03.009.吴柳, 张洁, 马文婷, 等. 下瘀血汤对胆碱蛋氨酸缺乏诱导的小鼠非酒精性脂肪性肝炎的抑制作用[J/CD]. 中国肝脏病杂志(电子版), 2018, 10( 3): 48- 55. DOI: 10.3969/j.issn.1674-7380.2018.03.009. [10] WU L, YANG GY, ZHANG J, et al. Xiayuxue decoction improved HFD-induced-nonalcoholic steatohepatitis mice by down-regulating NLRP3[J]. Chin J Integr Tradit West Med, 2020, 40( 10): 1202- 1208. DOI: 10.7661/j.cjim.20200904.336.吴柳, 杨广越, 张洁, 等. 下瘀血汤下调NLRP3改善高脂饮食诱导小鼠非酒精性脂肪性肝炎[J]. 中国中西医结合杂志, 2020, 40( 10): 1202- 1208. DOI: 10.7661/j.cjim.20200904.336. [11] HOU LQ, WANG ZY, ZHAO X, et al. Therapeutic effect and mechanism of Xiayuxue Decoction on mouse model of nonalcoholic fatty liver disease induced by high-fat diet[J]. J Clin Hepatol, 2024, 40( 4): 712- 719. DOI: 10.12449/JCH240412.侯林圻, 王知意, 赵鑫, 等. 下瘀血汤对高脂饮食诱导的非酒精性脂肪性肝病小鼠模型的治疗作用及机制[J]. 临床肝胆病杂志, 2024, 40( 4): 712- 719. DOI: 10.12449/JCH240412. [12] National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese Medical Association; Fatty Liver Expert Committee, Chinese Medical Doctor Association. Guidelines of prevention and treatment for nonalcoholic fatty liver disease: A 2018 update[J]. J Clin Hepatol, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007.中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34( 5): 947- 957. DOI: 10.3969/j.issn.1001-5256.2018.05.007. [13] TAO L, YANG GY, SUN TT, et al. Capsaicin receptor TRPV1 maintains quiescence of hepatic stellate cells in the liver via recruitment of SARM1[J]. J Hepatol, 2023, 78( 4): 805- 819. DOI: 10.1016/j.jhep.2022.12.031. [14] GAO JL, LI YH, ZHANG YJ, et al. Severity and remission of metabolic dysfunction-associated fatty/steatotic liver disease with chronic kidney disease occurrence[J]. J Am Heart Assoc, 2024, 13( 5): e032604. DOI: 10.1161/JAHA.123.032604. [15] OU FB, LUO SH, LI XF, et al. Metabolic-associated fatty liver disease as a driver of chronic kidney disease[J]. J Clin Exp Med, 2023, 22( 4): 443- 447. DOI: 10.3969/j.issn.1671-4695.2023.04.030.欧芳波, 罗沈晖, 李学锋, 等. 代谢相关脂肪性肝病:慢性肾脏病的一个启动因素[J]. 临床和实验医学杂志, 2023, 22( 4): 443- 447. DOI: 10.3969/j.issn.1671-4695.2023.04.030. [16] RATZIU V, HARRISON SA, LOUSTAUD-RATTI V, et al. Hepatic and renal improvements with FXR agonist vonafexor in individuals with suspected fibrotic NASH[J]. J Hepatol, 2023, 78( 3): 479- 492. DOI: 10.1016/j.jhep.2022.10.023. [17] WANG YH, CHEN WD, LI HM, et al. Application of the theory of“homology of liver and kidney” in the treatment of nonalcoholic fatty liver disease[J]. Chin J Integr Tradit West Med Liver Dis, 2023, 33( 8): 765- 768. DOI: 10.3969/j.issn.1005-0264.2023.008.023.王俞涵, 陈伟栋, 厉蕙萌, 等.“肝肾同源” 理论在非酒精性脂肪性肝病治疗中的应用[J]. 中西医结合肝病杂志, 2023, 33( 8): 765- 768. DOI: 10.3969/j.issn.1005-0264.2023.008.023. [18] QIU HT, YU XM. Syndrome differentiation and treatment of chronic nephropathy from hepatorenal homology[J]. Chin Med Mod Distance Educ China, 2023, 21( 5): 71- 74. DOI: 10.3969/j.issn.1672-2779.2023.05.027.邱海彤, 于秀梅. 从肝肾同源辨治慢性肾病[J]. 中国中医药现代远程教育, 2023, 21( 5): 71- 74. DOI: 10.3969/j.issn.1672-2779.2023.05.027. [19] CHEN P, LOU J. Discussion on nonalcoholic fatty liver disease with liver and kidney deficiency based on“homogeny of liver and kidney” theory[J]. Tradit Chin Med Res, 2022, 35( 12): 4- 8. DOI: 10.3969/j.issn.1001-6910.2022.12.02.陈萍, 娄静. 基于“肝肾同源” 理论探讨肝肾不足型非酒精性脂肪性肝病的治疗[J]. 中医研究, 2022, 35( 12): 4- 8. DOI: 10.3969/j.issn.1001-6910.2022.12.02. [20] Branch of Gastrointestinal Diseases, China Association of Chinese Medicine. Expert consensus on TCM diagnosis and treatment of nonalcoholic fatty liver disease(2017)[J]. J Clin Hepatol, 2017, 33( 12): 2270- 2274. DOI: 10.3969/j.issn.1001-5256.2017.12.002.中华中医药学会脾胃病分会. 非酒精性脂肪性肝病中医诊疗专家共识意见(2017)[J]. 临床肝胆病杂志, 2017, 33( 12): 2270- 2274. DOI: 10.3969/j.issn.1001-5256.2017.12.002. [21] Liver Disease Committee, Chinese Association of Integrative Medicine. Guidelines for the diagnosis and treatment of liver fibrosis in integrative medicine practice(2019)[J]. J Clin Hepatol, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007.中国中西医结合学会肝病专业委员会. 肝纤维化中西医结合诊疗指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 7): 1444- 1449. DOI: 10.3969/j.issn.1001-5256.2019.07.007. [22] LYU B, YANG MB, XIAO HB. Research on intervention mechanism of tanshinone type IIA sulfonate of adriamycin nephrosis rats kidney injury[J]. Chin Arch Tradit Chin Med, 2015, 33( 10): 2470- 2472, 26. DOI: 10.13193/j.issn.1673-7717.2015.10.051.吕波, 杨茂波, 肖洪彬. 丹参酮IIA磺酸钠对阿霉素肾病大鼠肾损伤的干预机制研究[J]. 中华中医药学刊, 2015, 33( 10): 2470- 2472, 26. DOI: 10.13193/j.issn.1673-7717.2015.10.051. [23] LIU Y, LI W. Pathogenesis and treatment of renal interstitial fibrosis based on collateral disease theory[J]. Chin J Basic Med Tradit Chin Med, 2019, 25( 11): 1521- 1524. DOI: 10.19945/j.cnki.issn.1006-3250.2019.11.015.刘瑶, 李伟. 基于络病理论的肾间质纤维化病机及治疗初探[J]. 中国中医基础医学杂志, 2019, 25( 11): 1521- 1524. DOI: 10.19945/j.cnki.issn.1006-3250.2019.11.015. [24] CORMICAN S, NEGI N, NAICKER SD, et al. Chronic kidney disease is characterized by expansion of a distinct proinflammatory intermediate monocyte subtype and by increased monocyte adhesion to endothelial cells[J]. J Am Soc Nephrol, 2023, 34( 5): 793- 808. DOI: 10.1681/ASN.0000000000000083. [25] LI XZ, BHATTACHARYA D, YUAN Y, et al. Chronic kidney disease in a murine model of non-alcoholic steatohepatitis(NASH)[J]. Kidney Int, 2024, 105( 3): 540- 561. DOI: 10.1016/j.kint.2023.12.009. -



 PDF下载 ( 3843 KB)
PDF下载 ( 3843 KB)

 下载:
下载: