索磷布韦/维帕他韦/伏西瑞韦治疗既往直接抗病毒药物治疗失败的慢性丙型肝炎患者的有效性和安全性
DOI: 10.12449/JCH241112
Efficacy and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous direct-acting antiviral agent failure
-
摘要:
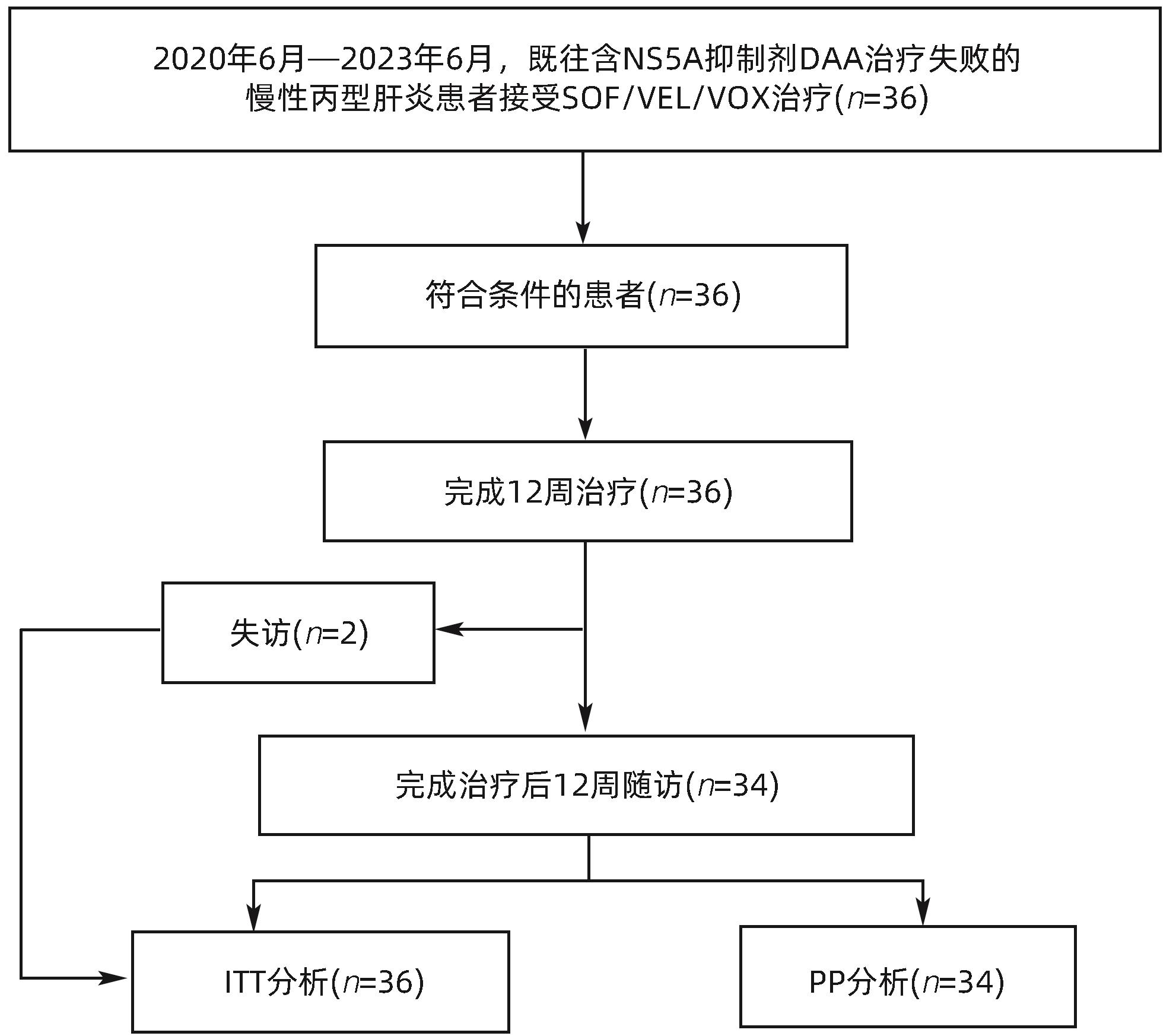
目的 评估真实世界中索磷布韦/维帕他韦/伏西瑞韦(SOF/VEL/VOX)用于既往接受直接抗病毒药物(DAA)治疗失败的慢性丙型肝炎患者的有效性和安全性。 方法 回顾性研究纳入2020年6月—2023年6月于南京市第二医院、无锡市第五人民医院和镇江市第三人民医院就诊的既往接受DAA抗病毒治疗失败并予以SOF/VEL/VOX(400 mg/100 mg/100 mg/片,1片/d)治疗12周的慢性丙型肝炎患者,观察治疗结束后12周持续病毒学应答(SVR12)情况,并评估生化学指标变化和不良反应发生情况,以评价药物的安全性。计量资料两组间比较采用配对t检验。 结果 共入组36例患者,非肝硬化27例,代偿期肝硬化9例,既往2次及以上DAA治疗失败4例。2例患者完成治疗后失访,余34例患者(94.4%)达到SVR12。入组的36例患者中,最常见的不良事件为瘙痒、恶心、疲劳和头痛;1例(2.78%)患者出现严重不良事件;未出现导致治疗药物停用或患者死亡的不良事件。 结论 SOF/VEL/VOX用于既往接受DAA治疗失败的丙型肝炎患者的挽救性治疗有较高的SVR12,且耐受性和安全性良好。 Abstract:Objective To investigate the efficacy and safety of sofosbuvir/velpatasivr/voxilaprevir (SOF/VEL/VOX) in patients with HCV infection experiencing failure in previous direct-acting antiviral agent (DAA) therapy. Methods A retrospective analysis was performed for the chronic hepatitis C patients who experienced failure in previous DAA antiviral therapy and were treated with SOF/VEL/VOX (400 mg/100 mg/100 mg/tablet, 1 tablet/day) for 12 weeks in Nanjing Second Hospital, Wuxi Fifth People’s Hospital, and The Third People’s Hospital of Zhenjiang from June 2020 to June 2023. Sustained virological response at 12 weeks (SVR12) was observed after the end of treatment, and the changes in biochemical parameters and the incidence rate of adverse reactions were assessed to evaluate drug safety. The paired t-test was used for comparison of continuous data between two groups. Results A total of 36 patients were enrolled, among whom there were 27 non-liver cirrhosis patients and 9 patients with compensated liver cirrhosis, and 4 patients experienced failure in the previous two or more sessions of DAA therapy. Two patients were lost to follow-up after treatment, and the remaining 34 patients (34/36, 94.4%) achieved SVR12. Among the 36 patients enrolled, the most common adverse events were pruritus, nausea, fatigue, and headache, and one patient (2.78%) experienced serious adverse events; there were no adverse events that resulted in the discontinuation of therapeutic agents or the death of patients. Conclusion For chronic hepatitis C patients who experience failure in previous DAA therapy, SOF/VEL/VOX salvage therapy has a relatively high rate of SVR12, with good tolerability and safety. -
Key words:
- Hepatitis C, Chronic /
- Salvage Therapy /
- Sofosbuvir /
- Velpatasvir /
- Voxilaprevir
-
表 1 患者基线特征和基因型分布
Table 1. Baseline characteristics and genotype distribution of patients
基线特征 数值 年龄(岁) 51.5±13.2 男[例(%)] 28(77.8) 既往DAA治疗失败次数[例(%)] 1 32(88.9) ≥2 4(11.1) 既往DAA治疗失败方案[例(%)] EBR/GZR 2(5.6) LDV/SOF 1(2.8) SOF+CLP 3(8.3) SOF/VEL 30(83.3) DAA挽救治疗方案[例(%)] SOF/VEL/VOX 31(86.1) SOF/VEL/VOX+RBV 5(13.9) 糖尿病[例(%)] 3(8.3) 高血压[例(%)] 5(13.9) 肝癌[例(%)] 2(5.6) 酒精性肝病[例(%)] 1(2.8) HCV RNA(log10IU/mL) 5.7±1.8 HCV基因型[例(%)] 1b 2(5.6) 3b 23(63.9) 6n 3(8.3) 混合基因型 3(8.3) 未知基因型 5(13.9) 注:EBR/GZR,艾尔巴韦/格拉瑞韦;LDV/SOF,来迪派韦/索磷布韦;SOF+CLP,索磷布韦+可洛派韦。
表 2 治疗前后生化指标变化
Table 2. Changes of biochemical indexes before and after treatment
指标 基线 治疗后12周 t值 P值 ALT(U/L) 63.21±30.33 26.64±7.62 2.86 <0.05 AST(U/L) 49.45±16.55 35.40±11.26 2.34 0.08 总胆红素(μmol/L) 13.72±5.68 18.24±6.27 -1.60 0.18 血小板计数(×109/L) 166.75±46.69 189.42±28.69 -2.89 <0.05 血红蛋白(g/L) 101.52±6.86 103.48±8.02 -2.06 0.11 血肌酐(μmol/L) 78.88±28.35 81.52±26.18 -0.22 0.84 白蛋白(g/L) 33.65±2.89 34.12±1.83 -0.29 0.79 表 3 SOF/VEL/VOX服药及随访期间的不良事件
Table 3. Adverse events during SOF/VEL/VOX treatment and follow-up
不良事件 例数(%) 任何不良事件 21(58.3) 严重不良事件 1(2.8) 死亡 0(0.0) ≥10%的不良事件 瘙痒 7(19.4) 恶心 5(13.9) 疲劳 4(11.1) 头痛 4(11.1) 3~4级不良事件 3(8.3) 因不良事件导致的治疗中断 0(0.0) -
[1] Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: A modelling study[J]. Lancet Gastroenterol Hepatol, 2022, 7( 5): 396- 415. DOI: 10.1016/S2468-1253(21)00472-6. [2] MANGIA A, MILLIGAN S, KHALILI M, et al. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts[J]. Liver Int, 2020, 40( 8): 1841- 1852. DOI: 10.1111/liv.14537. [3] XIAO L, ZHANG LY. Efficacy and safety of elbasvir/grazoprevir treatment for Chinese patients with hepatitis C virus genotype 1b[J]. Int J Virol, 2023, 30( 5): 423- 428. DOI: 10.3760/cma.j.issn.1673-4092.2023.05.017.肖琳, 张岭漪. 艾尔巴韦/格拉瑞韦治疗中国1b型慢性丙型肝炎患者的疗效和安全性[J]. 国际病毒学杂志, 2023, 30( 5): 423- 428. DOI: 10.3760/cma.j.issn.1673-4092.2023.05.017. [4] BOURLIÈRE M, GORDON SC, FLAMM SL, et al. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection[J]. N Engl J Med, 2017, 376( 22): 2134- 2146. DOI: 10.1056/NEJMoa1613512. [5] Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline for the prevention and treatment of hepatitis C(2022 version)[J]. Chin J Clin Infect Dis, 2022, 15( 6): 428- 447. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.002.中华医学会肝病学分会, 中华医学会感染病学分会. 丙型肝炎防治指南(2022年版)[J]. 中华临床感染病杂志, 2022, 15( 6): 428- 447. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.002. [6] European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series[J]. J Hepatol, 2020, 73( 5): 1170- 1218. DOI: 10.1016/j.jhep.2020.08.018. [7] RAO HY. Key points in hepatitis C guidance 2023 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection[J]. J Clin Hepatol, 2023, 39( 12): 2798- 2803. DOI: 10.3969/j.issn.1001-5256.2023.12.008.饶慧瑛.《美国肝病学会/美国感染病学会丙型肝炎指导意见: HCV感染的检测、管理和治疗(2023年更新)》意见要点[J]. 临床肝胆病杂志, 2023, 39( 12): 2798- 2803. DOI: 10.3969/j.issn.1001-5256.2023.12.008. [8] JI FP, LI J, LIU L, et al. High hepatitis C virus cure rates with approved interferon-free direct-acting antivirals among diverse mainland Chinese patients including genotypes 3a and 3b[J]. J Gastroenterol Hepatol, 2021, 36( 3): 767- 774. DOI: 10.1111/jgh.15192. [9] CHEN CY, HUANG CF, CHENG PN, et al. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan[J]. Liver Int, 2021, 41( 6): 1265- 1277. DOI: 10.1111/liv.14849. [10] MENDIZABAL M, PINERO F, RIDRUEJO E, et al. Disease progression in patients with hepatitis C virus infection treated with direct-acting antiviral agents[J]. Clin Gastroenterol Hepatol, 2020, 18( 11): 2554- 2563. e 3. DOI: 10.1016/j.cgh.2020.02.044. [11] CARRAT F, FONTAINE H, DORIVAL C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study[J]. Lancet, 2019, 393( 10179): 1453- 1464. DOI: 10.1016/S0140-6736(18)32111-1. [12] BACKUS LI, BELPERIO PS, SHAHOUMIAN TA, et al. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease[J]. Hepatology, 2019, 69( 2): 487- 497. DOI: 10.1002/hep.29408. [13] HONG CM, LIN YY, LIU CJ, et al. Drug resistance profile and clinical features for hepatitis C patients experiencing DAA failure in Taiwan[J]. Viruses, 2021, 13( 11): 2294. DOI: 10.3390/v13112294. [14] BELPERIO PS, SHAHOUMIAN TA, LOOMIS TP, et al. Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir in 573 direct-acting antiviral experienced hepatitis C patients[J]. J Viral Hepat, 2019, 26( 8): 980- 990. DOI: 10.1111/jvh.13115. [15] PAPALUCA T, ROBERTS SK, STRASSER SI, et al. Efficacy and safety of sofosbuvir/velpatasvir/voxilaprevir for hepatitis C virus(HCV) NS5A-inhibitor experienced patients with difficult to cure characteristics[J]. Clin Infect Dis, 2021, 73( 9): e3288- e3295. DOI: 10.1093/cid/ciaa1318. [16] LLANERAS J, RIVEIRO-BARCIELA M, LENS S, et al. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs[J]. J Hepatol, 2019, 71( 4): 666- 672. DOI: 10.1016/j.jhep.2019.06.002. [17] WILSON E, COVERT E, HOFFMANN J, et al. A pilot study of safety and efficacy of HCV retreatment with sofosbuvir/velpatasvir/voxilaprevir in patients with or without HIV(RESOLVE STUDY)[J]. J Hepatol, 2019, 71( 3): 498- 504. DOI: 10.1016/j.jhep.2019.05.021. [18] CHEN SS, YAN R, ZHOU K, et al. Efficacy of SOF/VEL/VOX retreatment in DAAs-failed chronic hepatitis C patients with different genotypes[J]. Chin J Clin Infect Dis, 2023, 16( 5): 372- 376. DOI: 10.3760/cma.j.issn.1674-2397.2023.05.006.陈闪闪, 严蓉, 周克, 等. 索磷布韦/维帕他韦/伏西瑞韦再治疗DAAs治疗失败的不同基因型慢性丙型肝炎患者的疗效分析[J]. 中华临床感染病杂志, 2023, 16( 5): 372- 376. DOI: 10.3760/cma.j.issn.1674-2397.2023.05.006. -



 PDF下载 ( 691 KB)
PDF下载 ( 691 KB)

 下载:
下载:


