三结构域蛋白29(TRIM29)与HBV复制及聚乙二醇干扰素α-2b抗病毒作用的关联性分析
DOI: 10.12449/JCH241111
Association of TRIM29 with HBV replication and the antiviral effect of pegylated interferon α-2b
-
摘要:
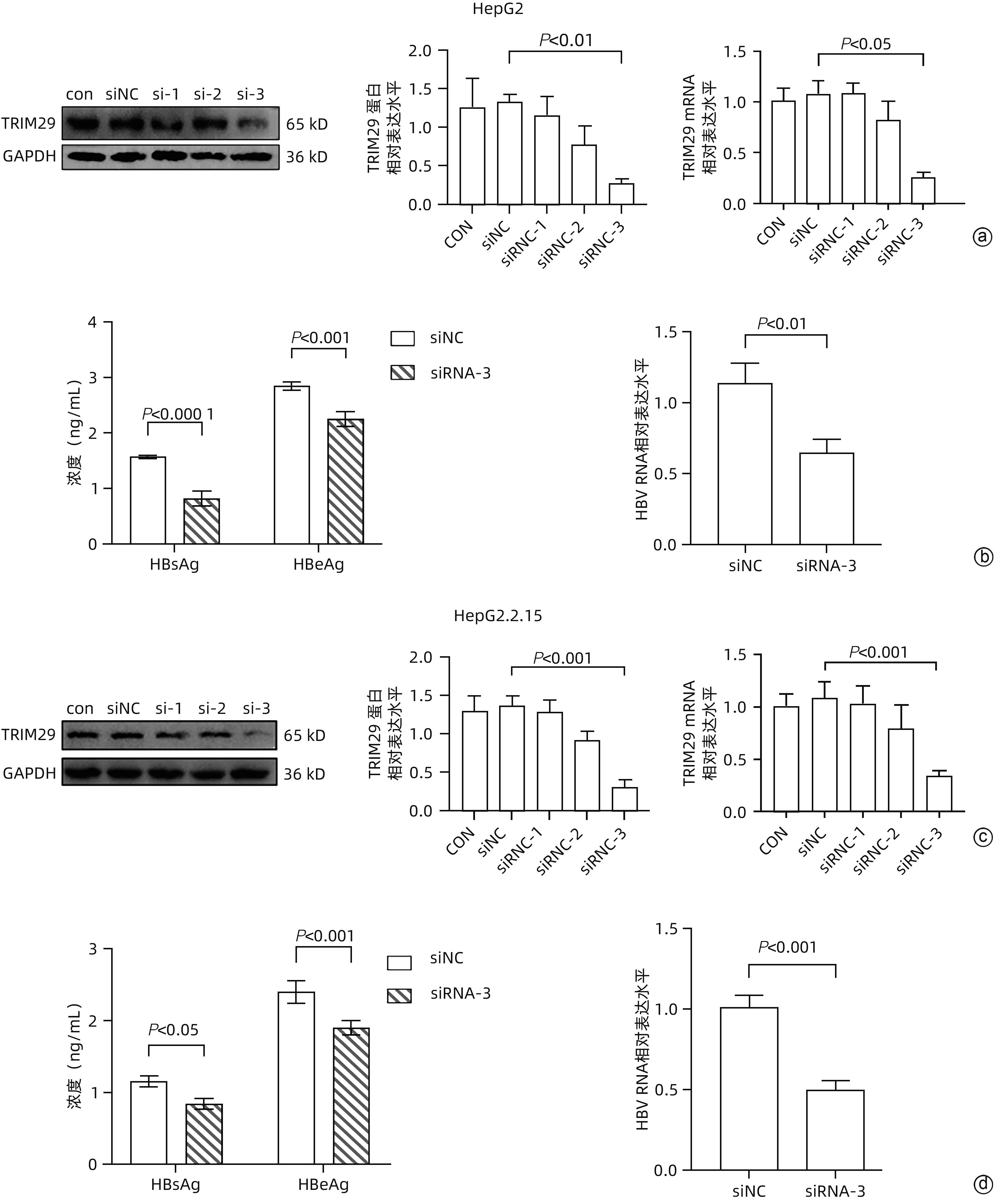
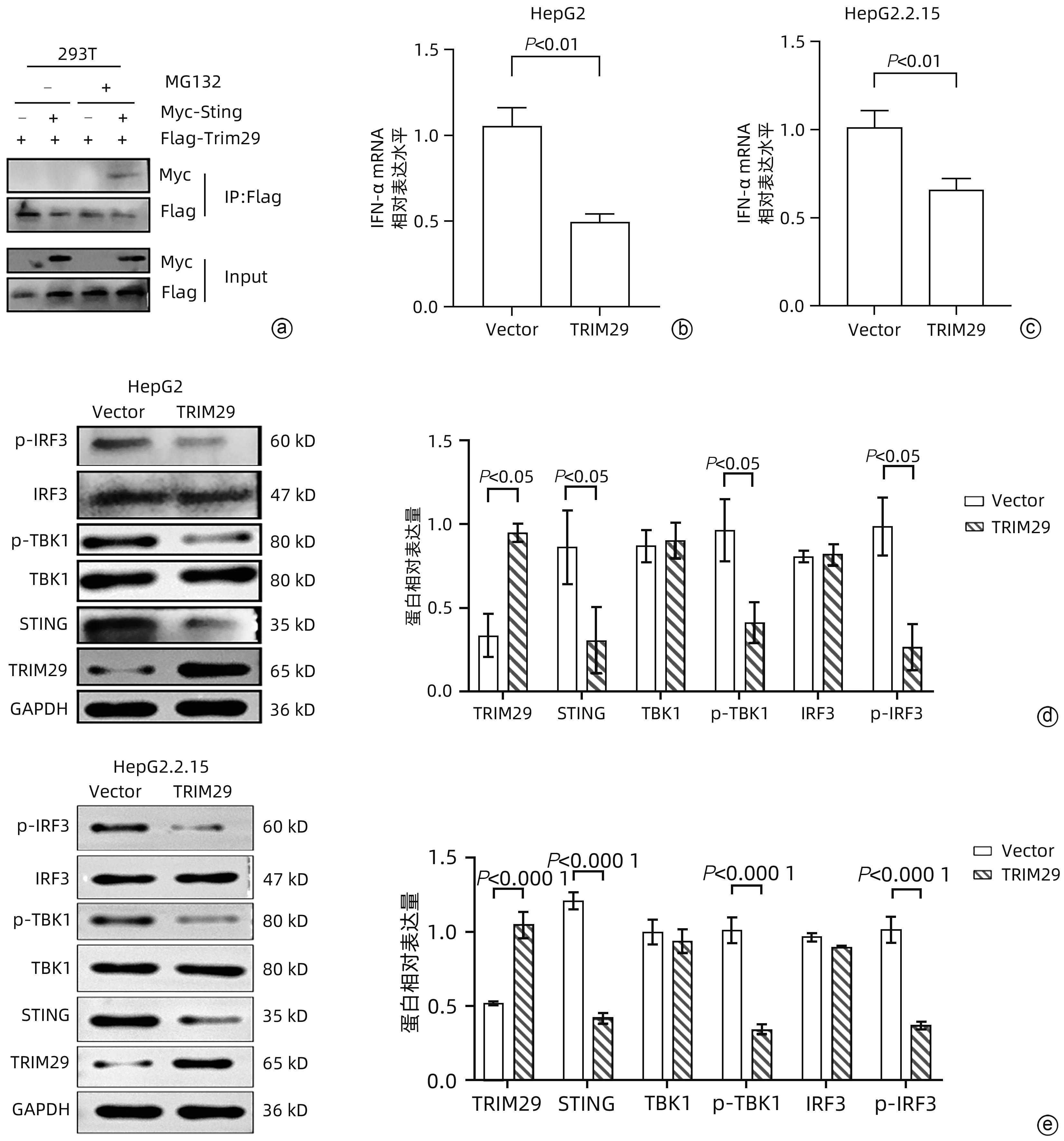
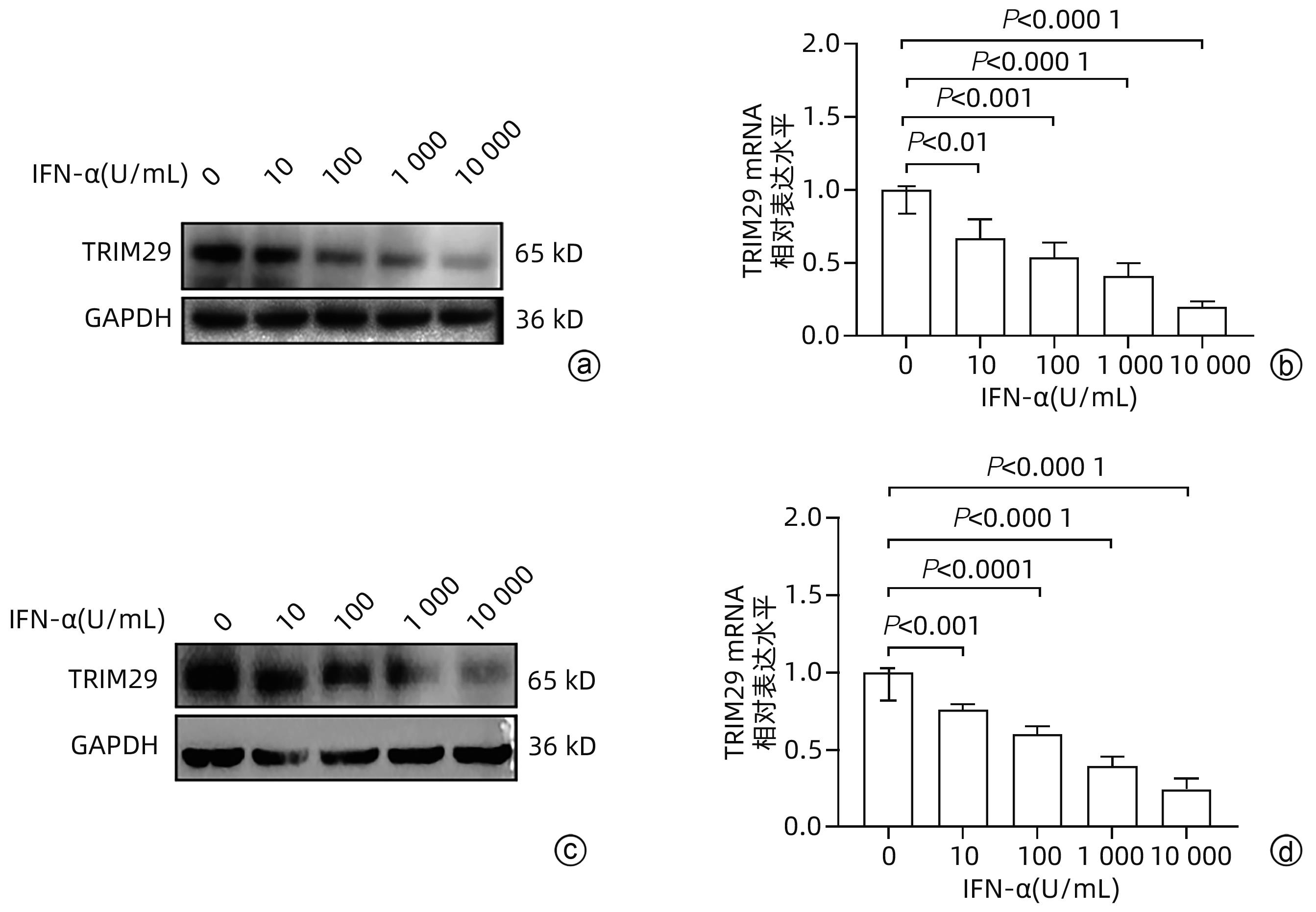
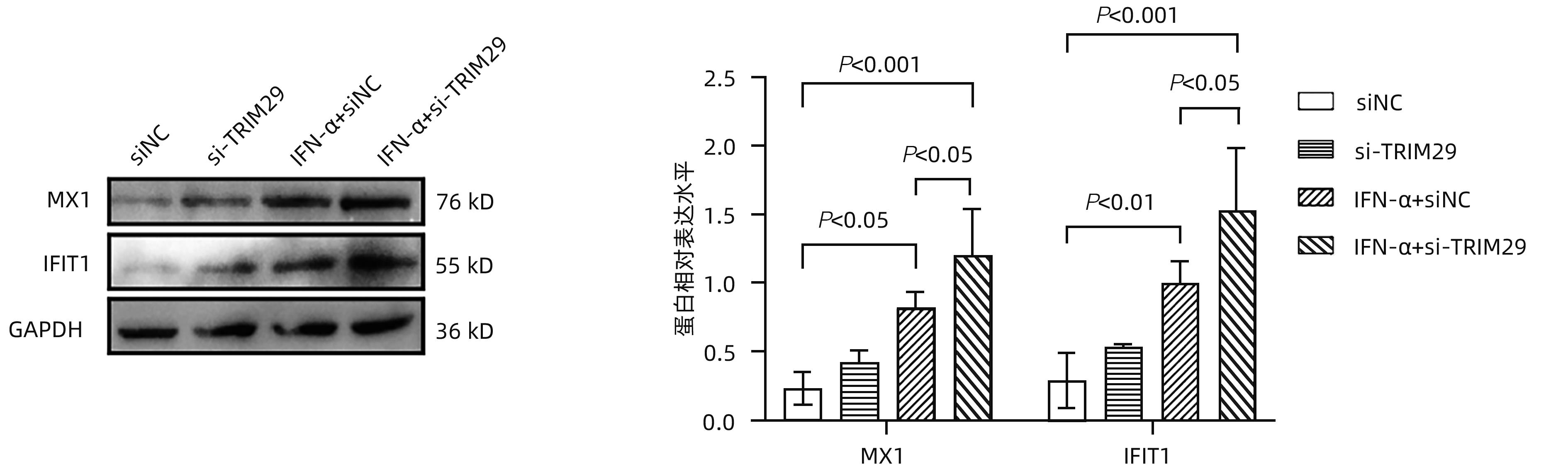
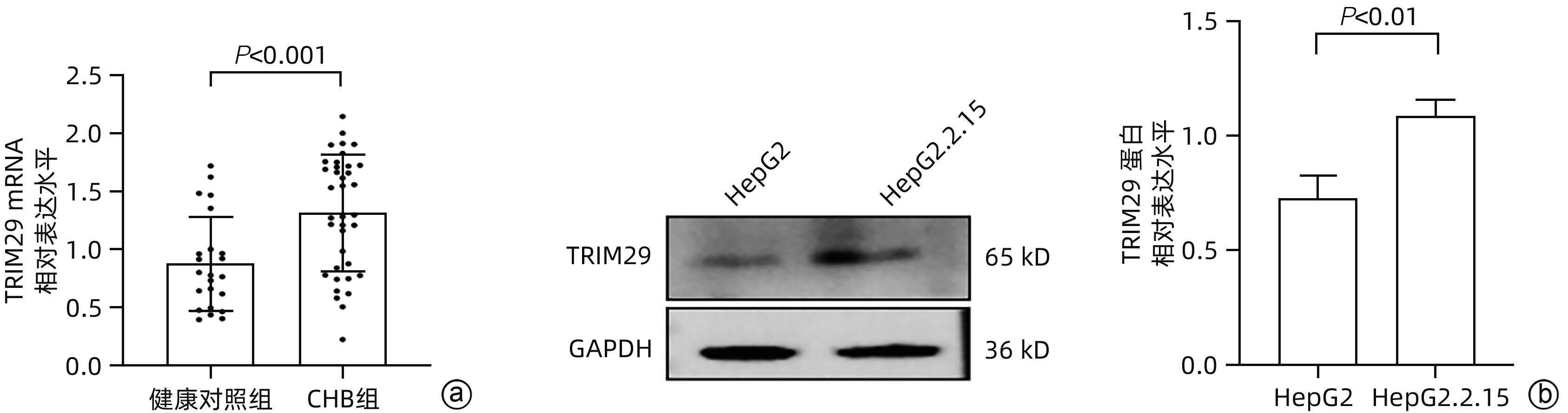
目的 三结构域蛋白29(TRIM29)参与多种疾病的发生和发展,与部分DNA和RNA病毒复制密切相关,本研究对TRIM29与HBV复制及聚乙二醇干扰素(PEG-IFN)α-2b抗病毒作用之间的关系展开初步讨论。 方法 选取2021年10月—2022年6月在贵州医科大学附属医院感染内科门诊就诊的CHB患者64例,其中未治疗患者34例(CHB组),经PEG-IFN-α- 2b治疗患者30例,体检中心30例健康志愿者作为对照(健康对照组)。收集志愿者年龄、性别、ALT、AST、TBil、DBil、HBV DNA和外周血单个核细胞(PBMC)。采用HepG2和HepG2.2.15细胞作为细胞模型,将TRIM29特异性过表达质粒或siRNA和对照转染至细胞;PEG-IFN-α-2b(0、10、100、1 000和10 000 U/mL)处理HepG2细胞和Huh7细胞;TRIM29特异性si-RNA或阴性对照联合PEG-IFN-α-2b处理HepG2.2.15细胞。ELISA检测HBsAg和HBeAg的浓度,qRT-PCR检测TRIM29和HBV RNA相对表达水平,Western Blot检测STING、p-TBK1、TBK1、pIRF3、IRF3、MX1和IFIT1蛋白表达情况,免疫共沉淀检测TRIM29与STING蛋白相互作用关系。正态分布的计量资料两组间比较采用成组t检验,多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验。计数资料两组间比较采用χ2检验或Fisher检验。 结果 CHB组患者外周血TRIM29表达明显高于健康对照组(P<0.001)。在细胞实验中,HBsAg、HBeAg和HBV RNA的表达均随TRIM29表达上调而升高,随TRIM29表达下调而降低(P值均<0.05)。TRIM29与STING互相结合,并通过蛋白酶降解STING,与对照组相比,过表达TRIM29对TBK1和IRF3总蛋白无明显变化,STING、p-TBK1和p-IRF3蛋白表达水平均降低(P值均<0.05)。处理HepG2细胞和Huh7细胞的PEG-IFN-α-2b浓度越高,TRIM29蛋白和mRNA的表达水平越低(P值均<0.01)。CHB患者在PEG-IFN-α-2b治疗期间,TRIM29 mRNA表达水平逐渐降低,且早期应答组和无应答组间差异均有统计学意义(P值均<0.05)。在等量PEG-IFN-α-2b处理下,与对照相比,敲低TRIM29后HepG2.2.15细胞的MX1和IFIT1蛋白表达水平明显增高(P值均<0.05)。在PEG-IFN-α-2b治疗早期,CHB患者PBMC中TRIM29表达逐渐降低。 结论 TRIM29靶向并降解STING,通过抑制STING-TBK1-IRF3信号通路促进HBV复制。TRIM29干扰PEG-IFN-α-2b的抗病毒作用,CHB患者PBMC中TRIM29的表达水平可能作为预测患者对PEG-IFN-α-2b治疗应答的指标。 Abstract:Objective To preliminarily investigate the association of TRIM29 with HBV replication and the antiviral effect of pegylated interferon α-2b (PEG-IFN-α-2b), since TRIM29 protein is involved in the development and progression of a variety of diseases and is closely associated with the replication of some DNA and RNA viruses. Methods A total of 64 chronic hepatitis B (CHB) patients who attended the outpatient service of Department of Infectious Diseases, The Affiliated Hospital of Guizhou Medical University, from October 2021 to June 2022 were enrolled, among whom there were 34 treatment-naïve patients and 30 patients treated with PEG-IFN-α-2b, and 30 healthy volunteers in Physical Examination Center were enrolled as controls. Related data were collected, including age, sex, alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin, HBV DNA, and peripheral blood mononuclear cells (PBMCs). HepG2 and HepG2.2.15 cells were used as cell models and were transfected with TRIM29-specific overexpressed plasmid or siRNA and control plasmid. HepG2 cells and Huh7 cells were treated with PEG-IFN-α-2b (0, 10, 100, 1 000, and 10 000 U/mL), and HepG2.2.15 cells were treated with TRIM29-specific siRNA or negative control combined with PEG-IFN-α-2b. ELISA was used to measure the concentrations of HBsAg and HBeAg; qRT-PCR was used to measure the relative expression levels of TRIM29 and HBV RNA; Western blot was used to measure the protein expression levels of STING, p-TBK1, TBK1, pIRF3, IRF3, MX1, and IFIT1; co-immunoprecipitation assay was used to observe the interaction between TRIM29 and STING protein. The independent-samples t test was used for comparison of normally distributed continuous data between two groups, and a one-way analysis of variance was used for comparison between multiple groups, with the least significant difference t-test for further comparison between two groups; the chi-square test or the Fisher’s exact test was used for comparison of categorical data between two groups. Results The CHB patients had a significantly higher expression level of TRIM29 in peripheral blood than the healthy controls (P<0.001). In cell experiments, the expression levels of HBsAg, HBeAg, and HBV RNA increased with the upregulation of TRIM29 expression and decreased with downregulation of TRIM29 expression (P<0.05). TRIM29 bound to STING and degraded STING via protease, and compared with the control group, there were no significant changes in the total protein levels of TBK1 and IRF3 after overexpression of TRIM29, while there were significant reductions in the expression levels of STING, p-TBK1, and p-IRF3 (P<0.05). The protein and mRNA expression levels of TRIM29 decreased with the increase in the concentration of PEG-IFN-α-2b for the treatment of HepG2 and Huh7 cells (P<0.01). During the treatment with PEG-IFN-α-2b, the CHB patients had a gradual reduction in the mRNA expression level of TRIM29, and there was a significant difference between the early response group and the non-response group (P<0.05). In the context of treatment with an equal volume of PEG-IFN-α-2b, compared with the control group, there were significant increases in the protein expression levels of Mx1 and IFIT1 in HepG2.2.15 cells after TRIM29 knockdown (P<0.05). There was a gradual reduction in the expression of TRIM29 in CHB patients during the early stage of PEG-IFN-α-2b treatment. Conclusion TRIM29 targets and degrades STING and promotes HBV replication by inhibiting the STING-TBK1-IRF3 signaling pathway. TRIM29 interferes with the antiviral effect of PEG-IFN-α-2b, and the expression level of TRIM29 in PBMCs of CHB patients may be used as an indicator for predicting the response of patients to PEG-IFN-α-2b therapy. -
Key words:
- Hepatitis B Virus /
- Tripartite Motif-Containing Protein 29 /
- PEG-IFNα /
- Signaling Pathway
-
表 1 引物序列
Table 1. List of primers
引物 序列(5'-3') HBV RNA-F TCTTGCCTTACTTTTGGAAG HBV RNA-R AGTTCTTCTTCTAGGGGACC Hu-TRIM29-F TGCGAGCTGCATCTCAAGC Hu-TRIM29-R GGTGCTATGATTCTTGTGCTCC Hu-IFN-α-F TGACCTCAAAGCCTGTGTGATG Hu-IFN-α-R AAGTATTTCCTCACAGCCAGCAG Hu-GAPDH-F CGGATTTGGTCGTATTGGG Hu-GAPDH-R TCTCGCTCCTGGAAGATGG 表 2 健康对照组和CHB组患者的一般资料比较
Table 2. Comparison of clinical characteristics between healthy and CHB patients
指标 健康对照组(n=30) CHB组(n=34) 统计值 P值 年龄(岁) 38.2±10.4 33.8±5.0 t=0.451 0.328 男/女(例) 15/15 16/18 χ2=0.055 0.814 汉族/其他(例) 23/7 24/10 χ2=0.302 0.583 ALT(U/L) 19.4±4.8 17.4±4.9 t=1.702 0.094 AST(U/L) 17.3±3.3 18.4±3.3 t=-1.293 0.201 TBil(μmol/L) 6.2±3.7 8.6±2.7 t=-1.791 0.195 DBil(μmol/L) 3.4±0.8 4.6±1.6 t=-2.219 0.138 表 3 一般临床资料
Table 3. Clinical characteristics of patients
指标 早期应答组(n=11) 早期无应答组(n=19) t值 P值 女性(例) 7 12 >0.05 年龄(岁) 26.8±4.49 29.3±7.20 -2.041 0.643 HBV DNA (log10 IU/mL) 0周 2.35±0.26 2.11±0.18 2.380 0.010 12周 1.33±0.10 1.84±0.23 2.598 <0.001 24周 1.09±0.16 1.56±0.27 -4.260 <0.001 ALT(U/L) 0周 23.64±3.70 24.09±4.00 -0.546 0.99 12周 59.78±6.30 36.25±5.73 8.127 <0.001 24周 33.66±6.74 45.68±4.96 -3.893 <0.001 TRIM29 mRNA 0周 1.57±0.43 1.32±0.75 2.07 0.047 12周 1.22±0.26 0.72±0.34 3.83 <0.001 24周 0.94±0.27 0.43±0.11 7.20 <0.001 -
[1] JENG WJ, PAPATHEODORIDIS GV, LOK ASF. Hepatitis B[J]. Lancet, 2023, 401( 10381): 1039- 1052. DOI: 10.1016/s0140-6736(22)01468-4. [2] LIU YS, CHEN XY. Advances in the research and development of new drugs for chronic hepatitis B[J]. J Clin Hepatol, 2022, 38( 6): 1387- 1392. DOI: 10.3969/j.issn.1001-5256.2022.06.035.刘义思, 陈新月. 治疗慢性乙型肝炎新药研发的研究进展[J]. 临床肝胆病杂志, 2022, 38( 6): 1387- 1392. DOI: 10.3969/j.issn.1001-5256.2022.06.035. [3] van GENT M, SPARRER KMJ, GACK MU. TRIM proteins and their roles in antiviral host defenses[J]. Annu Rev Virol, 2018, 5( 1): 385- 405. DOI: 10.1146/annurev-virology-092917-043323. [4] MERONI G. Genomics and evolution of the TRIM gene family[J]. Adv Exp Med Biol, 2012, 770: 1- 9. DOI: 10.1007/978-1-4614-5398-7_1. [5] JAWORSKA AM, WLODARCZYK NA, MACKIEWICZ A, et al. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal[J]. Stem Cells, 2020, 38( 2): 165- 173. DOI: 10.1002/stem.3109. [6] LEONHARDT EA, KAPP LN, YOUNG BR, et al. Nucleotide sequence analysis of a candidate gene for ataxia-telangiectasia group D(ATDC)[J]. Genomics, 1994, 19( 1): 130- 136. DOI: 10.1006/geno.1994.1022. [7] KAPP LN, PAINTER RB, YU LC, et al. Cloning of a candidate gene for ataxia-telangiectasia group D[J]. Am J Hum Genet, 1992, 51( 1): 45- 54. [8] QIAO HY, ZHANG Q, WANG JM, et al. TRIM29 regulates the SETBP1/SET/PP2A axis via transcription factor VEZF1 to promote progression of ovarian cancer[J]. Cancer Lett, 2022, 529: 85- 99. DOI: 10.1016/j.canlet.2021.12.029. [9] SUN JT, ZHANG TY, CHENG MM, et al. Correction to: TRIM29 facilitates the epithelial-tomesenchymal transition and the progression of colorectal cancer via the activation of the Wnt/β-catenin signaling pathway[J]. J Exp Clin Cancer Res, 2021, 40( 1): 145. DOI: 10.1186/s13046-021-01922-w. [10] DU HM, XU Q, XIAO S, et al. MicroRNA-424-5p acts as a potential biomarker and inhibits proliferation and invasion in hepatocellular carcinoma by targeting TRIM29[J]. Life Sci, 2019, 224: 1- 11. DOI: 10.1016/j.lfs.2019.03.028. [11] XING JJ, ZHANG A, MINZE LJ, et al. TRIM29 negatively regulates the type I IFN production in response to RNA virus[J]. J Immunol, 2018, 201( 1): 183- 192. DOI: 10.4049/jimmunol.1701569. [12] HATAKEYAMA S. TRIM proteins and cancer[J]. Nat Rev Cancer, 2011, 11( 11): 792- 804. DOI: 10.1038/nrc3139. [13] Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.王贵强, 王福生, 庄辉, 等. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [14] YAN H, ZHONG GC, XU GW, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus[J]. Elife, 2012, 1: e00049. DOI: 10.7554/eLife.00049. [15] MARTINEZ MG, BOYD A, COMBE E, et al. Covalently closed circular DNA: The ultimate therapeutic target for curing HBV infections[J]. J Hepatol, 2021, 75( 3): 706- 717. DOI: 10.1016/j.jhep.2021.05.013. [16] TIAN YJ, CHEN WL, KUO CF, et al. Viral-load-dependent effects of liver injury and regeneration on hepatitis B virus replication in mice[J]. J Virol, 2012, 86( 18): 9599- 9605. DOI: 10.1128/JVI.01087-12. [17] LUCIFORA J, XIA YC, REISINGER F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA[J]. Science, 2014, 343( 6176): 1221- 1228. DOI: 10.1126/science.1243462. [18] XIA YC, STADLER D, LUCIFORA J, et al. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis[J]. Gastroenterology, 2016, 150( 1): 194- 205. DOI: 10.1053/j.gastro.2015.09.026. [19] TROPBERGER P, MERCIER A, ROBINSON M, et al. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation[J]. Proc Natl Acad Sci U S A, 2015, 112( 42): E5715- E5724. DOI: 10.1073/pnas.1518090112. [20] MARTINS-DE-SOUZA D, GATTAZ WF, SCHMITT A, et al. Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia[J]. Eur Arch Psychiatry Clin Neurosci, 2009, 259( 3): 151- 163. DOI: 10.1007/s00406-008-0847-2. [21] NAKAGAMI H, KIKUCHI Y, KATSUYA T, et al. Gene polymorphism of myospryn(cardiomyopathy-associated 5) is associated with left ventricular wall thickness in patients with hypertension[J]. Hypertens Res, 2007, 30( 12): 1239- 1246. DOI: 10.1291/hypres.30.1239. [22] CAMBIAGHI V, GIULIANI V, LOMBARDI S, et al. TRIM proteins in cancer[J]. Adv Exp Med Biol, 2012, 770: 77- 91. DOI: 10.1007/978-1-4614-5398-7_6. [23] STREMLAU M, OWENS CM, PERRON MJ, et al. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys[J]. Nature, 2004, 427( 6977): 848- 853. DOI: 10.1038/nature02343. [24] SONG YH, LI M, WANG YQ, et al. E3 ubiquitin ligase TRIM21 restricts hepatitis B virus replication by targeting HBx for proteasomal degradation[J]. Antiviral Res, 2021, 192: 105107. DOI: 10.1016/j.antiviral.2021.105107. [25] TAN GY, YI ZH, SONG HX, et al. Type-I-IFN-stimulated gene TRIM5γ inhibits HBV replication by promoting HBx degradation[J]. Cell Rep, 2019, 29( 11): 3551- 3563. e 3. DOI: 10.1016/j.celrep.2019.11.041. [26] SONG HX, XIAO QF, XU FC, et al. TRIM25 inhibits HBV replication by promoting HBx degradation and the RIG-I-mediated pgRNA recognition[J]. Chin Med J(Engl), 2023, 136( 7): 799- 806. DOI: 10.1097/CM9.0000000000002617. [27] ZHOU JL, ZHUANG Z, LI JM, et al. Significance of the cGAS-STING pathway in health and disease[J]. Int J Mol Sci, 2023, 24( 17): 13316. DOI: 10.3390/ijms241713316. [28] CHEN BJ, RAO XY, WANG XY, et al. cGAS-STING signaling pathway and liver disease: From basic research to clinical practice[J]. Front Pharmacol, 2021, 12: 719644. DOI: 10.3389/fphar.2021.719644. [29] WU JJ, DOBBS N, YANG K, et al. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion[J]. Immunity, 2020, 53( 1): 115- 126. e 5. DOI: 10.1016/j.immuni.2020.06.009. [30] DECOUT A, KATZ JD, VENKATRAMAN S, et al. The cGAS-STING pathway as a therapeutic target in inflammatory diseases[J]. Nat Rev Immunol, 2021, 21( 9): 548- 569. DOI: 10.1038/s41577-021-00524-z. [31] LIU SQ, CAI X, WU JX, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation[J]. Science, 2015, 347( 6227): aaa2630. DOI: 10.1126/science.aaa2630. [32] LI Q, LIN L, TONG Y, et al. TRIM29 negatively controls antiviral immune response through targeting STING for degradation[J]. Cell Discov, 2018, 4: 13. DOI: 10.1038/s41421-018-0010-9. [33] JANSSEN HLA, van ZONNEVELD M, SENTURK H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: A randomised trial[J]. Lancet, 2005, 365( 9454): 123- 129. DOI: 10.1016/S0140-6736(05)17701-0. [34] XIE F, XIONG X, YAO CX, et al. Clinical efficacy and influencing factors of pegylated interferon alfa-2b and nucleos(t)ide analogue in chronic hepatitis B patients with low level of hepatitis B virus surface antigen[J/CD]. Chin J Exp Clin Infect Dis(Electronic Edition), 2022, 16( 4): 247- 253. DOI: 10.3877/cma.j.issn.1674-1358. 2022. 04. 005.谢芳, 熊熙, 姚传霞, 等. 聚乙二醇化干扰素α-2b联合核苷(酸)类似物治疗低水平乙型肝炎病毒表面抗原慢性乙型肝炎患者的临床疗效及影响因素[J/CD]. 中华实验和临床感染病杂志(电子版), 2022, 16( 4): 247- 253. DOI: 10.3877/cma.j.issn.1674-1358. 2022. 04. 005. [35] YE JY, CHEN JL. Interferon and hepatitis B: Current and future perspectives[J]. Front Immunol, 2021, 12: 733364. DOI: 10.3389/fimmu.2021.733364. [36] XU BF, TANG B, WEI JJ. Role of STAT1 in the resistance of HBV to IFN-α[J]. Exp Ther Med, 2021, 21( 6): 550. DOI: 10.3892/etm.2021.9982. [37] REHERMANN B, BERTOLETTI A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections[J]. Hepatology, 2015, 61( 2): 712- 721. DOI: 10.1002/hep.27323. [38] LUO MQ, HOU J, MAI HM, et al. TRIM26 inhibits hepatitis B virus replication by promoting HBx degradation and TRIM26 genetic polymorphism predicts PegIFNα treatment response of HBeAg-positive chronic hepatitis B Patients[J]. Aliment Pharmacol Ther, 2022, 56( 5): 878- 889. DOI: 10.1111/apt.17124. -



 PDF下载 ( 1873 KB)
PDF下载 ( 1873 KB)

 下载:
下载: