求同存异,兼容并济——从国内外慢加急性肝衰竭定义的演变历程统一共识
DOI: 10.12449/JCH241108
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:徐曼曼负责撰写论文;陈煜负责拟定写作思路,指导论文撰写并修改论文。
Unified consensus and evolution of the definition of acute-on-chronic liver failure: Seeking common ground while reserving differences
-
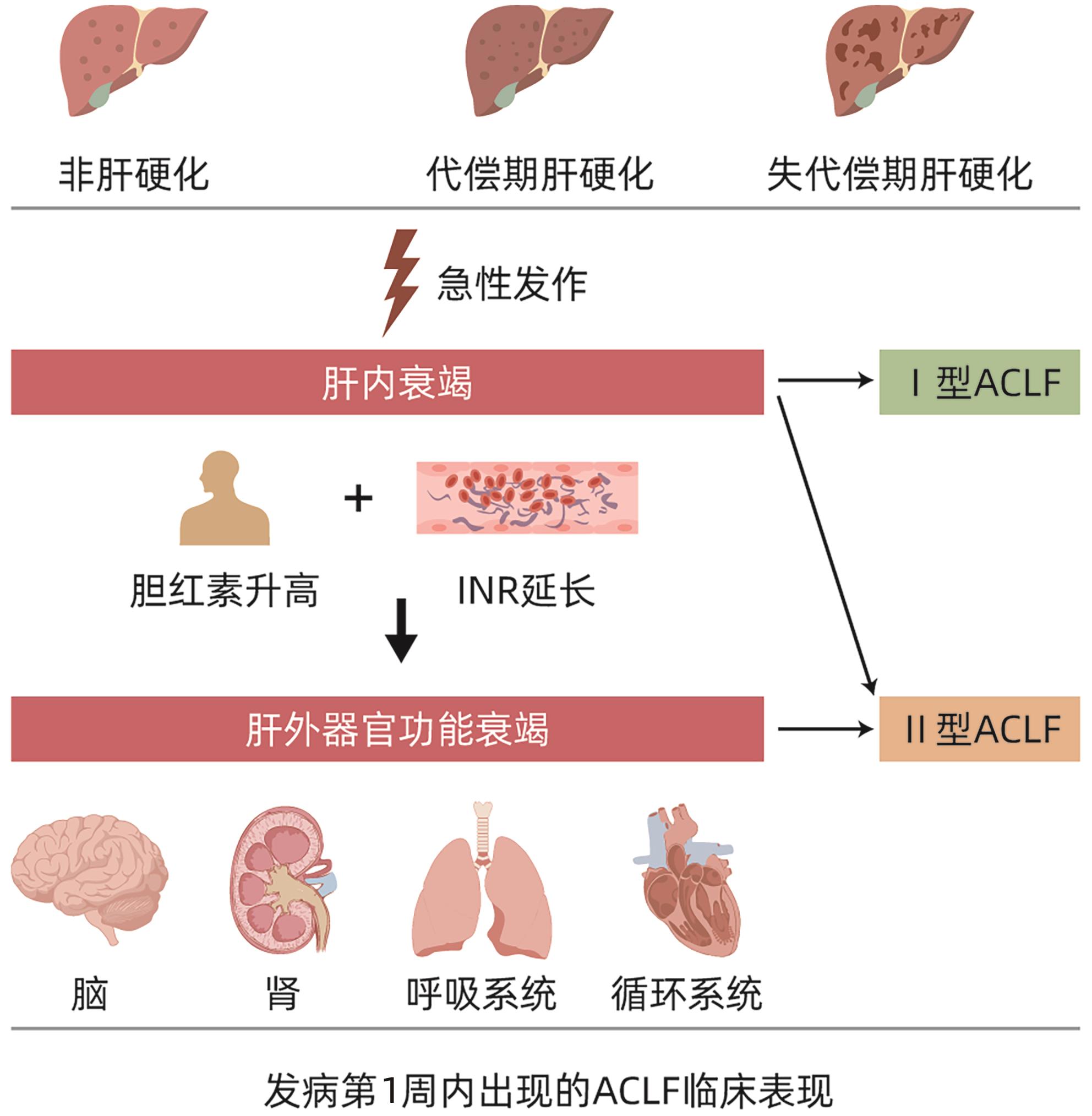
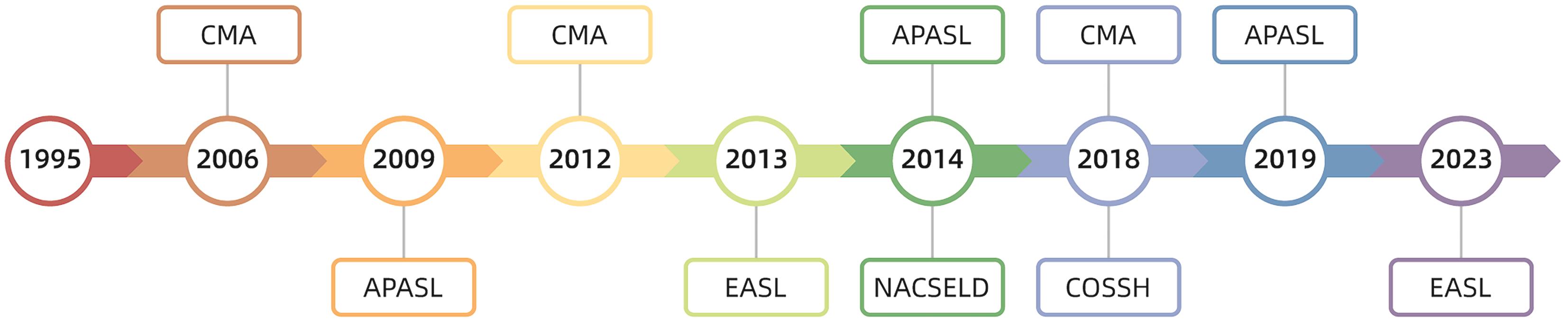
摘要: 在慢加急性肝衰竭(ACLF)这一概念被提出的近30年来,随着对其发病机制、诊断标准、治疗措施等各个方面研究的不断深入,来自全球范围内的相关诊疗共识指南也在不断更新,但ACLF的定义始终未得到统一,定义的不同必然会阻碍诸多治疗手段、预后评分、诊疗意见的推广应用。近年来,肝病学专家们不断提出了统一ACLF定义的方式方法,求同存异,取长补短,最终力求使ACLF的定义趋于一致。Abstract: The concept of acute-on-chronic liver failure (ACLF) has been introduced for nearly 30 years, and with extensive research on its pathogenesis, diagnostic criteria, and treatment strategies, related consensus statements and guidelines have been constantly updated in China and globally; however, there is still a lack of a unified definition of ACLF, and such differences in definition may inevitably hinder the application and implementation of various treatment methods, prognostic scoring systems, and clinical recommendations. In recent years, hepatology experts have continuously proposed methods to unify the definition of ACLF, seeking common ground while reserving differences and drawing on the strengths of various definitions, in order to achieve a more unified definition of ACLF.
-
Key words:
- Acute-on-Chronic Liver Failure /
- Definition /
- Evolution
-
-
[1] OHNISHI H, SUGIHARA J, MORIWAKI H, et al. Acute-on-chronic liver failure[J]. Ryoikibetsu Shokogun Shirizu, 1995( 7): 217- 219. [2] SARIN SK, KUMAR A, ALMEIDA JA, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL)[J]. Hepatol Int, 2009, 3( 1): 269- 282. DOI: 10.1007/s12072-008-9106-x. [3] SARIN SK, KEDARISETTY CK, ABBAS Z, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver(APASL) 2014[J]. Hepatol Int, 2014, 8( 4): 453- 471. DOI: 10.1007/s12072-014-9580-2. [4] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [5] JALAN R, SALIBA F, PAVESI M, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure[J]. J Hepatol, 2014, 61( 5): 1038- 1047. DOI: 10.1016/j.jhep.2014.06.012. [6] BAJAJ JS, O’LEARY JG, REDDY KR, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures[J]. Hepatology, 2014, 60( 1): 250- 256. DOI: 10.1002/hep.27077. [7] WU TZ, LI J, SHAO L, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure[J]. Gut, 2018, 67( 12): 2181- 2191. DOI: 10.1136/gutjnl-2017-314641. [8] KIMMANN M, TREBICKA J. Acute-on-chronic liver failure: Current interventional treatment options and future challenges[J]. J Pers Med, 2023, 13( 7): 1052. DOI: 10.3390/jpm13071052. [9] LI YH, XU Y, WU HM, et al. Umbilical cord-derived mesenchymal stem cell transplantation in hepatitis B virus related acute-on-chronic liver failure treated with plasma exchange and entecavir: A 24-month prospective study[J]. Stem Cell Rev Rep, 2016, 12( 6): 645- 653. DOI: 10.1007/s12015-016-9683-3. [10] WANG HM, YAO WQ, WANG YY, et al. Meta-analysis on last ten years of clinical injection of bone marrow-derived and umbilical cord MSC to reverse cirrhosis or rescue patients with acute-on-chronic liver failure[J]. Stem Cell Res Ther, 2023, 14( 1): 267. DOI: 10.1186/s13287-023-03494-2. [11] SALIBA F, BAÑARES R, LARSEN FS, et al. Artificial liver support in patients with liver failure: A modified DELPHI consensus of international experts[J]. Intensive Care Med, 2022, 48( 10): 1352- 1367. DOI: 10.1007/s00134-022-06802-1. [12] LARSEN FS. Artificial liver support in acute and acute-on-chronic liver failure[J]. Curr Opin Crit Care, 2019, 25( 2): 187- 191. DOI: 10.1097/MCC.0000000000000584. [13] SAHA BK, MAHTAB M AL, AKBAR SMF, et al. Therapeutic implications of granulocyte colony stimulating factor in patients with acute-on-chronic liver failure: Increased survival and containment of liver damage[J]. Hepatol Int, 2017, 11( 6): 540- 546. DOI: 10.1007/s12072-017-9814-1. [14] ENGELMANN C, HERBER A, FRANKE A, et al. Granulocyte-colony stimulating factor(G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial(GRAFT study)[J]. J Hepatol, 2021, 75( 6): 1346- 1354. DOI: 10.1016/j.jhep.2021.07.033. [15] PATEL A, WALLING A, KANWAL F, et al. Rates, patterns, and predictors of specialty palliative care consultation among patients with acute-on-chronic liver failure[J]. JHEP Rep, 2023, 6( 2): 100976. DOI: 10.1016/j.jhepr.2023.100976. [16] PERRICONE G, ARTZNER T, de MARTIN E, et al. Intensive care management of acute-on-chronic liver failure[J]. Intensive Care Med, 2023, 49( 8): 903- 921. DOI: 10.1007/s00134-023-07149-x. [17] HE WP, HU JH, ZHAO J, et al. Comparison of four prognostic models and a new Logistic regression model to predict short-term prognosis of acute-on-chronic hepatitis B liver failure[J]. Chin Med J(Engl), 2012, 125( 13): 2272- 2278. [18] JALAN R, YURDAYDIN C, BAJAJ JS, et al. Toward an improved definition of acute-on-chronic liver failure[J]. Gastroenterology, 2014, 147( 1): 4- 10. DOI: 10.1053/j.gastro.2014.05.005. [19] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [20] BAJAJ JS, O’LEARY JG, LAI JC, et al. Acute-on-chronic liver failure clinical guidelines[J]. Am J Gastroenterol, 2022, 117( 2): 225- 252. DOI: 10.14309/ajg.0000000000001595. [21] XU M, CHEN Y. New perspectives in the definition and classification of acute-on-chronic liver failure[J]. Chin Med J(Engl), 2024. DOI: 10.1097/CM9.0000000000003289.[ Online ahead of print]. [22] DONG JL, CHEN Y. Recognition of the clinical classification of acute-on-chronic liver failure: Redefinition from a new perspective of onset manifestations and dynamic outcomes[J]. J Clin Hepatol, 2023, 39( 10): 2277- 2280. DOI: 10.3969/j.issn.1001-5256.2023.10.002.董金玲, 陈煜. 慢加急性肝衰竭临床分型的再认识: 从起病表现和动态转归的新视角重新定义[J]. 临床肝胆病杂志, 2023, 39( 10): 2277- 2280. DOI: 10.3969/j.issn.1001-5256.2023.10.002. [23] XU MM, CHEN Y, ARTRU F. Acute decompensation of cirrhosis versus acute-on-chronic liver failure: What are the clinical implications?[J]. United European Gastroenterol J, 2024, 12( 2): 194- 202. DOI: 10.1002/ueg2.12538. [24] WU Y, DONG JL, XU MM, et al. Acute-on-chronic liver failure: Features and prognosis of a new clinical classification system based on onset manifestations[J]. J Clin Hepatol, 2023, 39( 10): 2375- 2382. DOI: 10.3969/j.issn.1001-5256.2023.10.015.武羽, 董金玲, 徐曼曼, 等. 慢加急性肝衰竭: 基于起病表现的新型临床分型特征及预后分析[J]. 临床肝胆病杂志, 2023, 39( 10): 2375- 2382. DOI: 10.3969/j.issn.1001-5256.2023.10.015. [25] KARVELLAS CJ, BAJAJ JS, KAMATH PS, et al. AASLD Practice Guidance on Acute-on-chronic liver failure and the management of critically ill patients with cirrhosis[J]. Hepatology, 2024, 79( 6): 1463- 1502. DOI: 10.1097/HEP.0000000000000671. [26] KULKARNI AV, SARIN SK. Acute-on-chronic liver failure-steps towards harmonization of the definition![J]. J Hepatol, 2024, 81( 2): 360- 366. DOI: 10.1016/j.jhep.2024.03.036. -



 PDF下载 ( 1061 KB)
PDF下载 ( 1061 KB)

 下载:
下载: