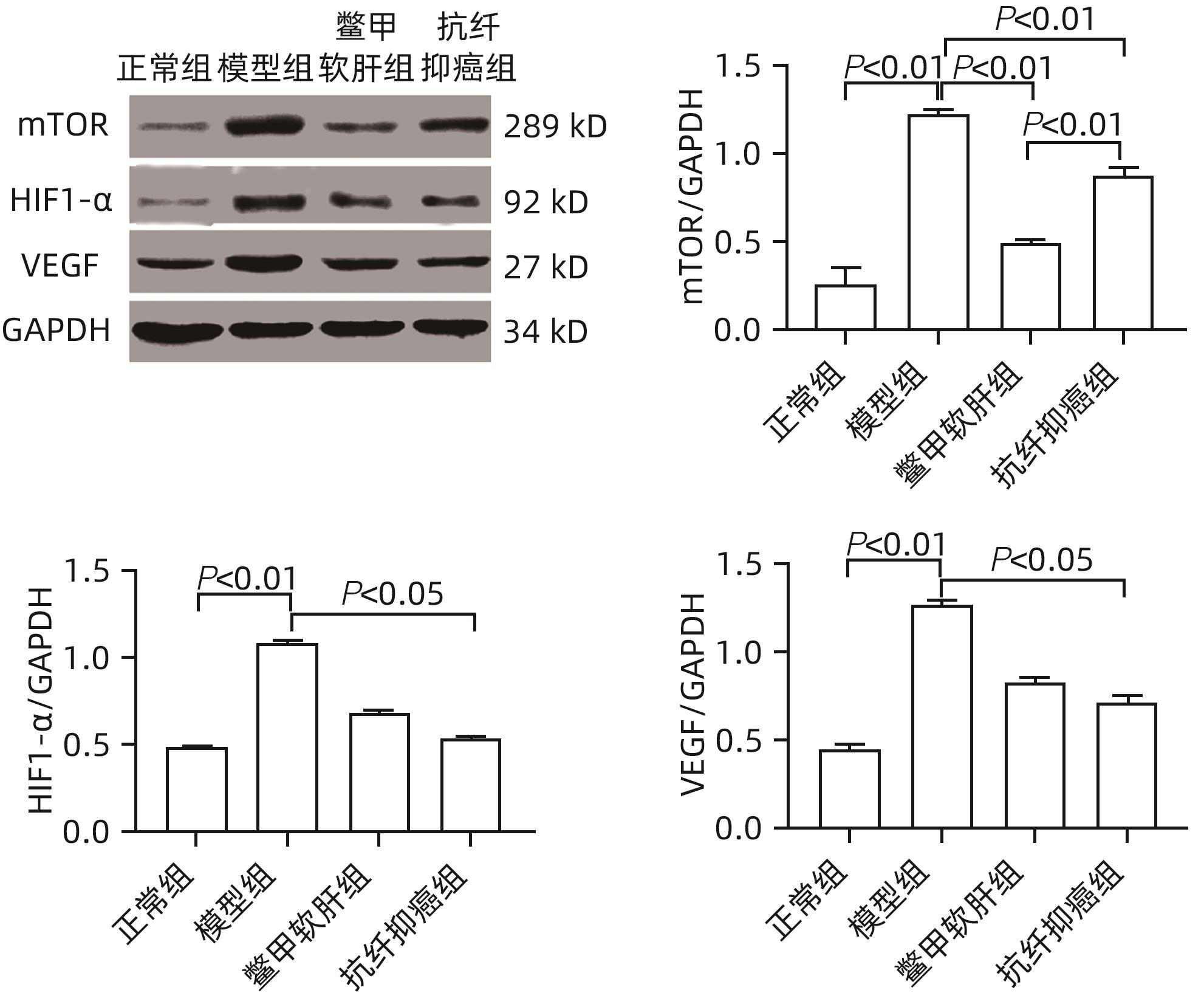
基于mTOR/HIF-1α/VEGF信号通路探讨抗纤抑癌方对肝癌前病变大鼠模型的调控作用
DOI: 10.12449/JCH241019
Regulatory effect of Kangxian Yiai Prescription in a rat model of precancerous lesions of liver cancer: A study based on the mTOR/HIF-1α/VEGF signaling pathway
-
摘要:
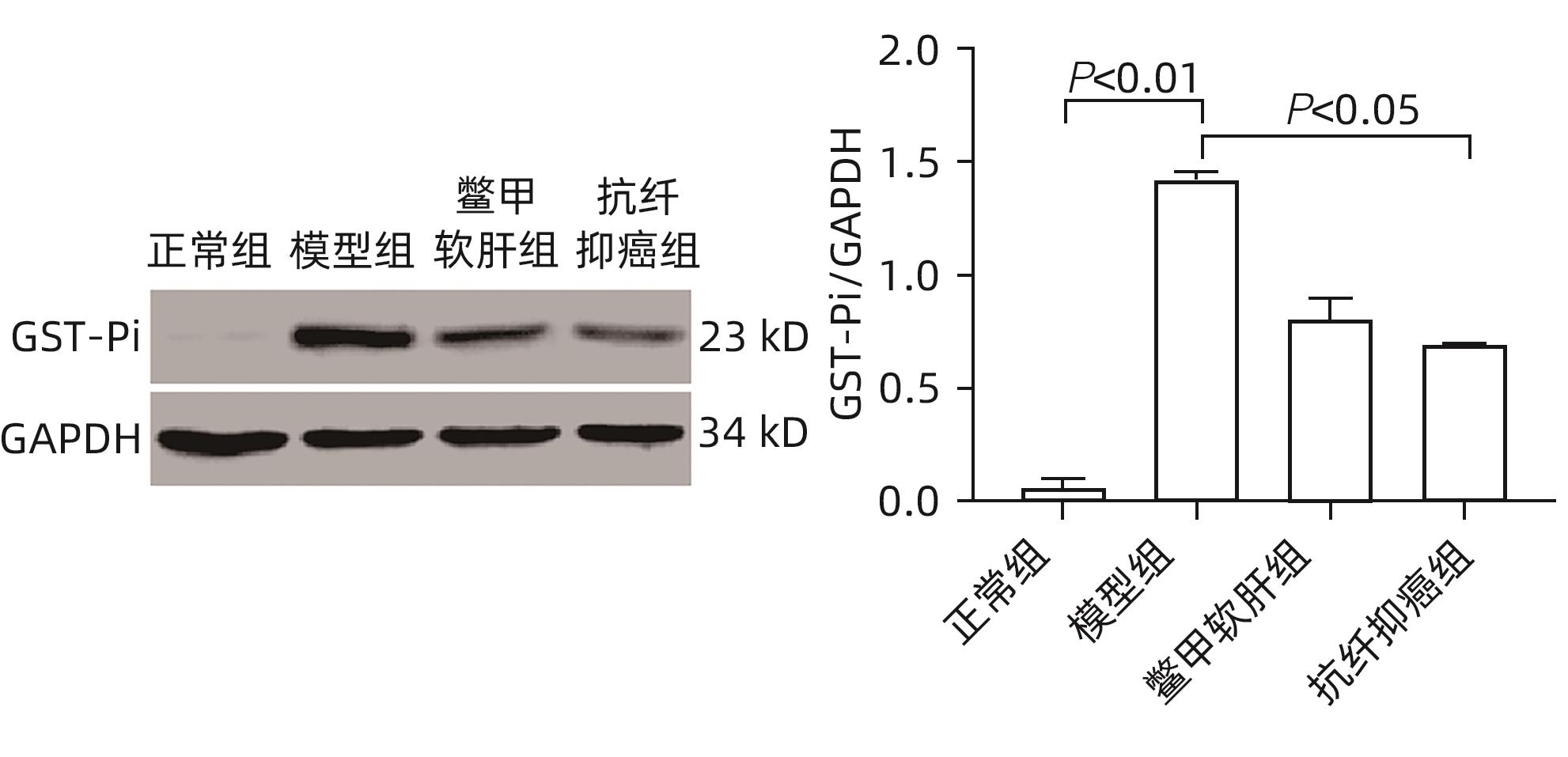
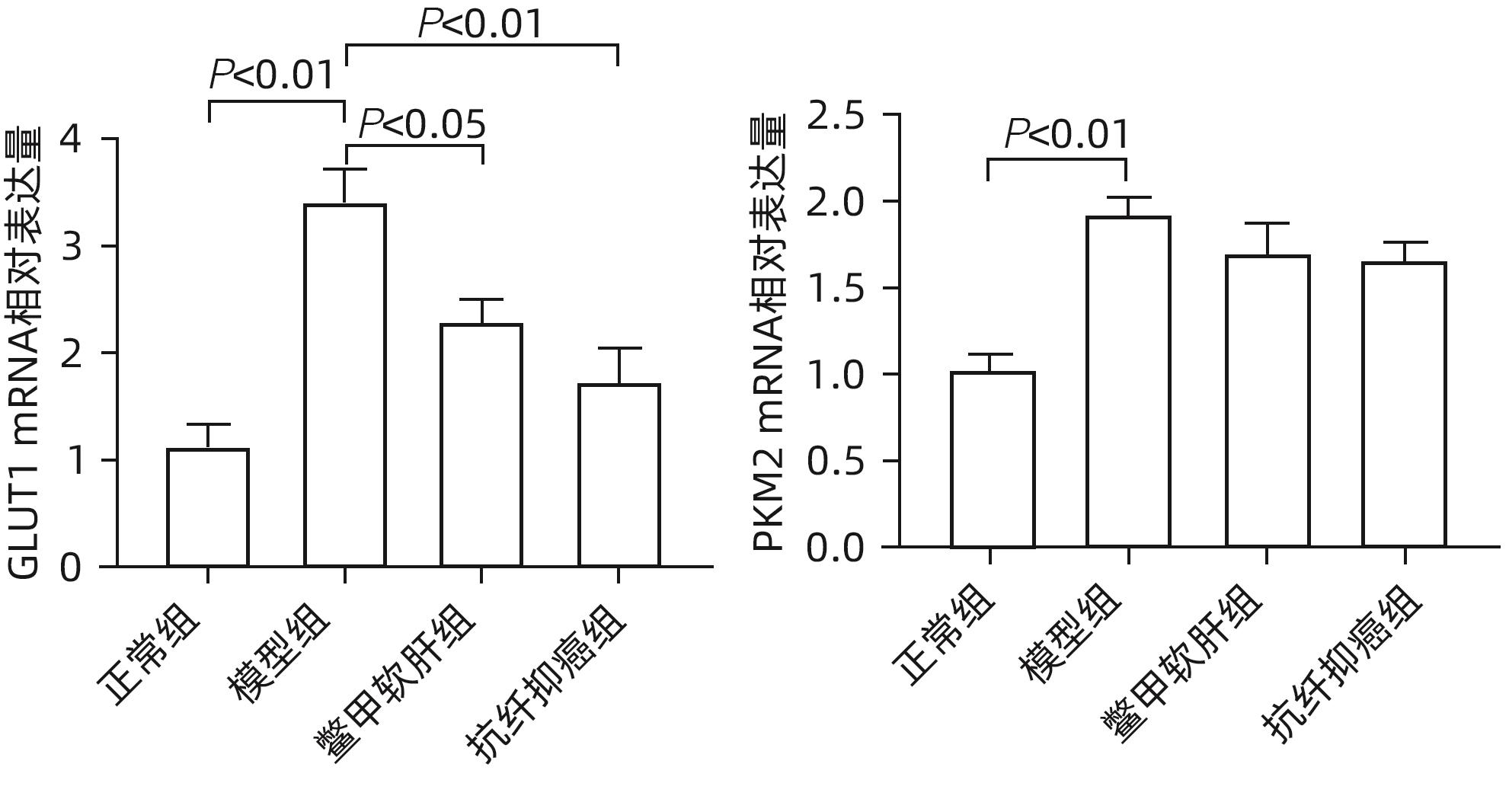
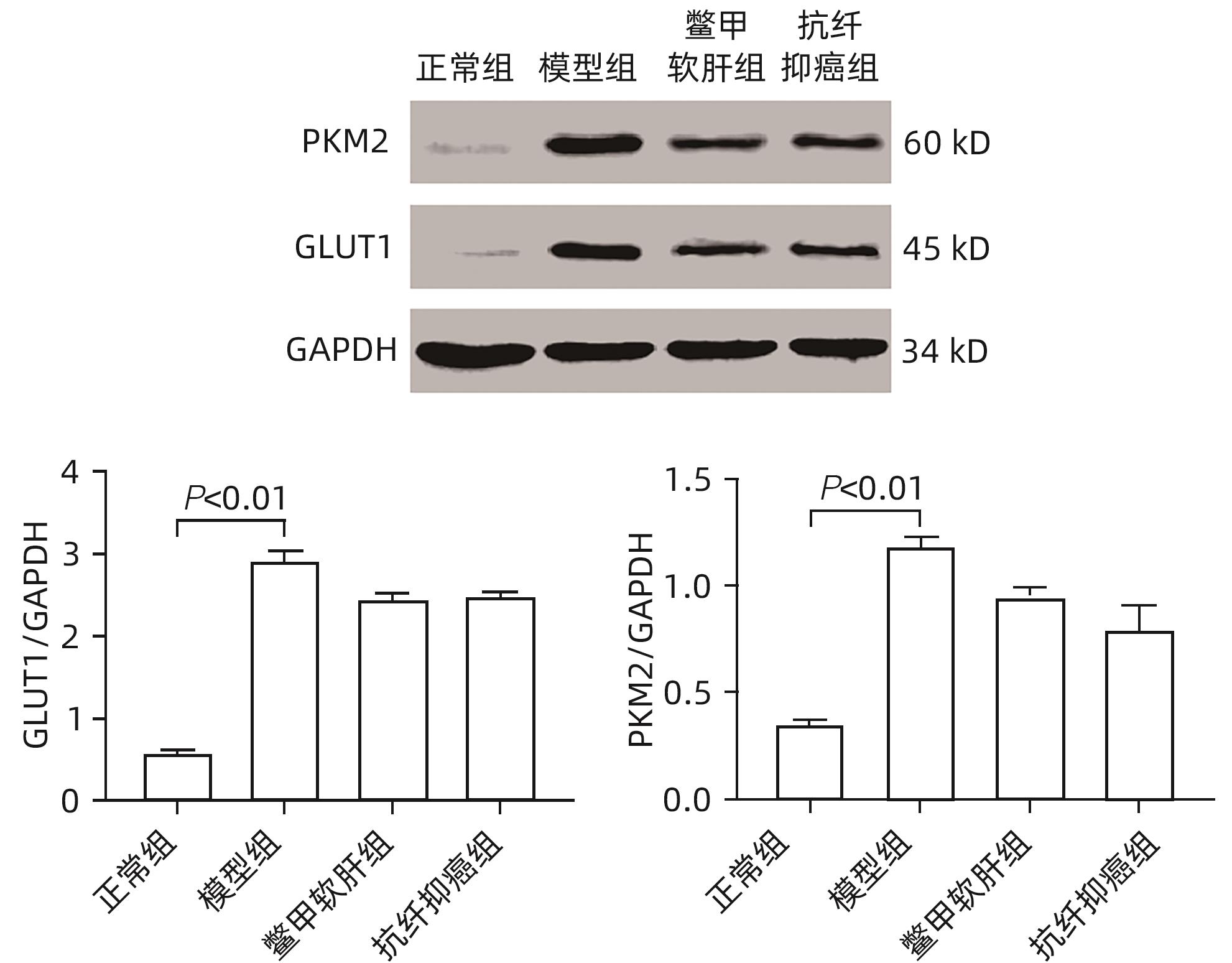
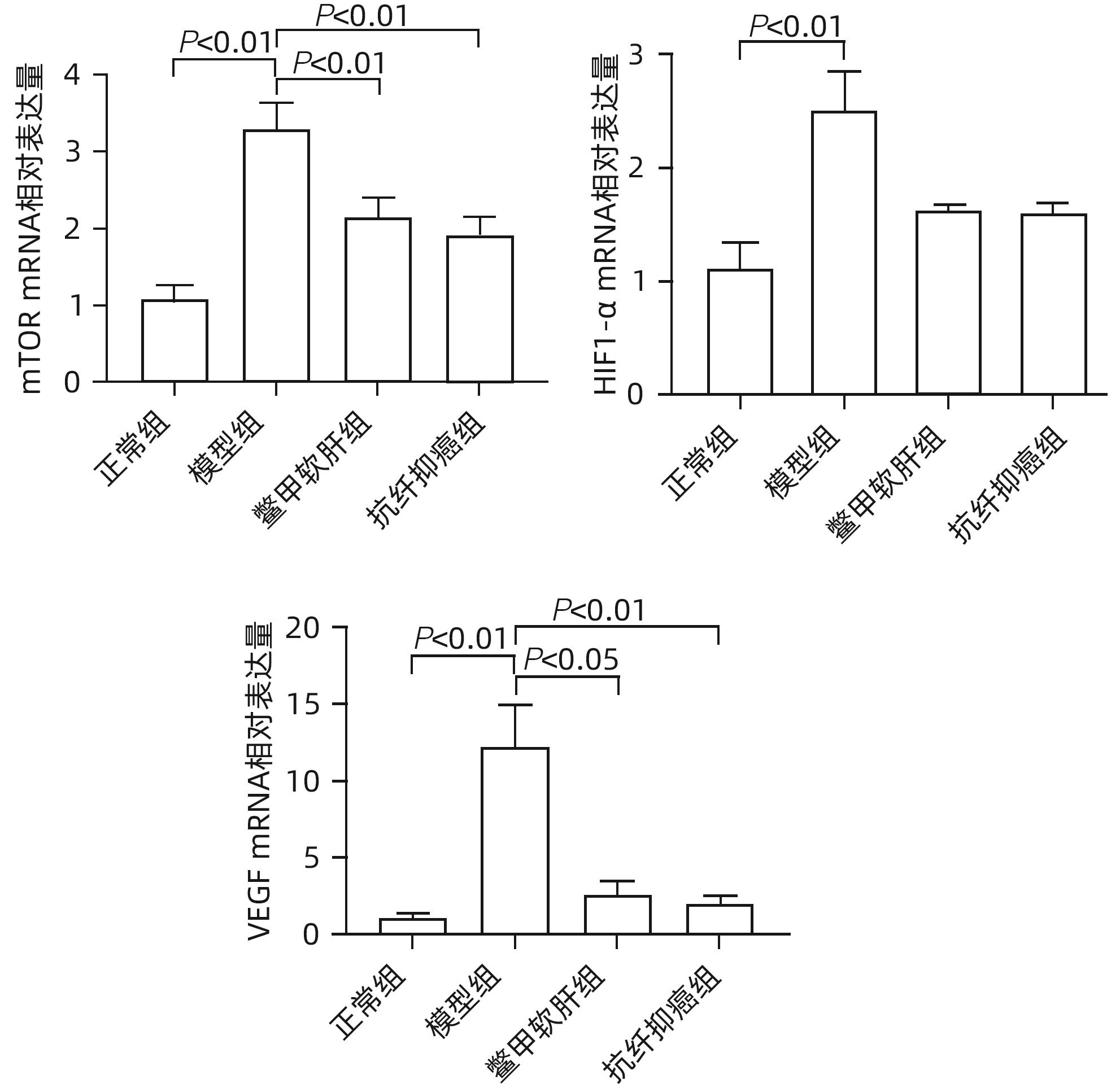
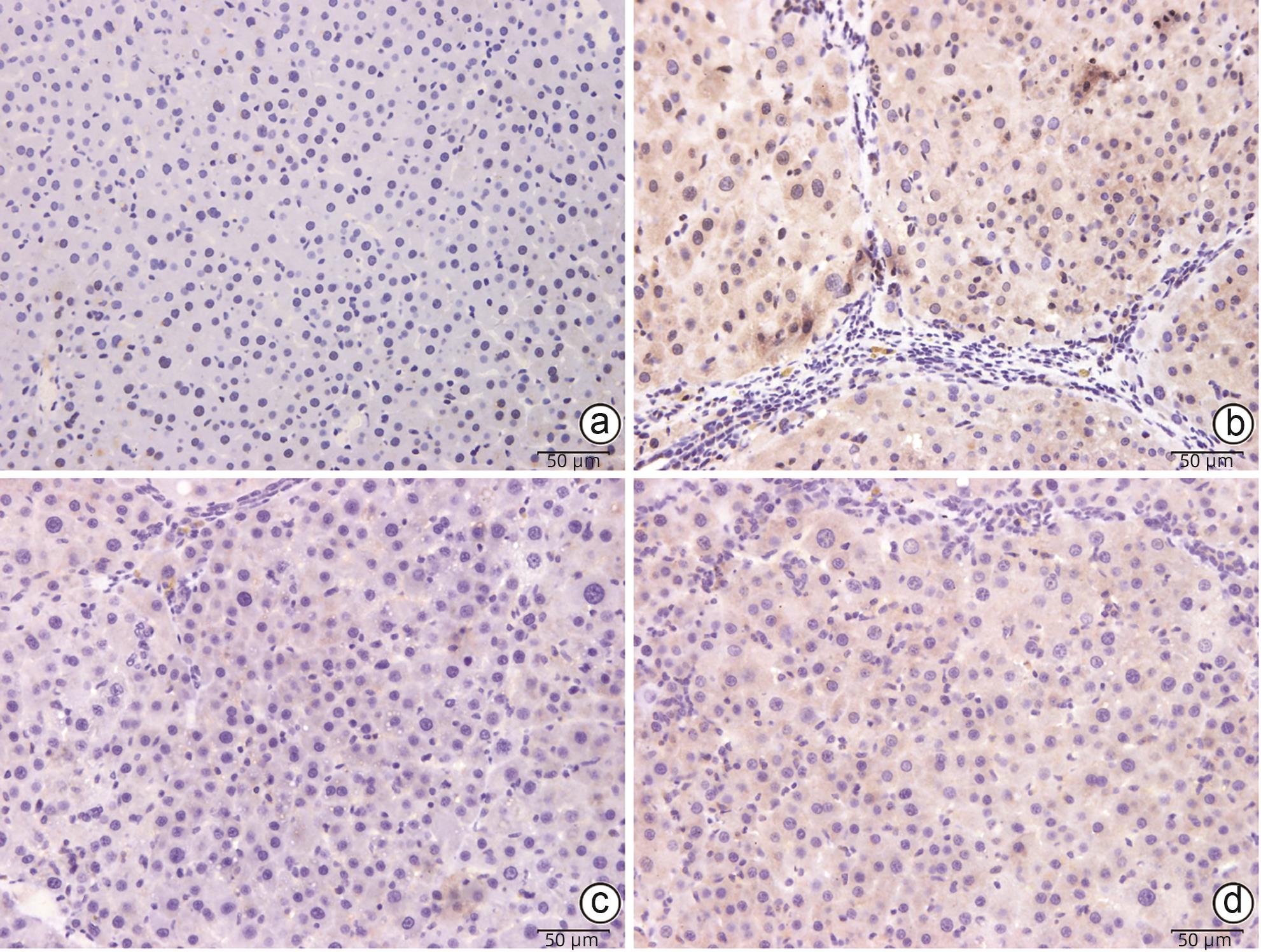
目的 研究抗纤抑癌方对肝癌前病变大鼠模型mTOR/HIF-1α/VEGF信号通路的影响。 方法 40只雄性Wistar大鼠,分为正常组、模型组、抗纤抑癌组和鳖甲软肝组,每组各10只。正常组大鼠腹腔注射生理盐水,剂量为0.4 mL/100 g,其他3组大鼠以50 mg/kg剂量腹腔注射二乙基亚硝胺,制备肝癌前病变大鼠模型。通过免疫组化和Western Blot法检测GST-Pi的表达,实时荧光定量PCR和Western Blot法检测mTOR、HIF-1α、VEGF、M2型丙酮酸激酶(PKM2)、葡萄糖载体蛋白1(GLUT-1) mRNA及蛋白的表达。计量资料多组间比较采用单因素方差分析或Kruskal-Wallis H秩和检验,进一步两两比较采用LSD-t检验。 结果 与正常组比较,模型组大鼠肝组织GST-Pi蛋白表达显著升高(P<0.01);与模型组比较,抗纤抑癌组GST-Pi蛋白表达水平显著降低(P<0.05)。与正常组比较,模型组大鼠肝组织GLUT1及PKM2 mRNA的表达均显著升高(P值均<0.01);与模型组比较,鳖甲软肝组及抗纤抑癌组GLUT1 mRNA的表达均显著降低(P值均<0.05)。与正常组比较,模型组大鼠肝组织GLUT1及PKM2的蛋白表达均显著升高(P值均<0.01)。与正常组比较,模型组大鼠肝组织mTOR、HIF-1α及VEGF的mRNA表达均显著升高(P值均<0.01);与模型组比较,鳖甲软肝组mTOR及VEGF的mRNA的表达均显著降低(P值均<0.05),抗纤抑癌组mTOR及VEGF mRNA的表达亦显著降低(P值均<0.01)。与正常组比较,模型组大鼠肝组织mTOR、HIF-1α、VEGF的蛋白表达均显著升高(P值均<0.01);与模型组相比,鳖甲软肝组只有mTOR的蛋白表达显著降低(P<0.01),抗纤抑癌组mTOR、HIF-1α、VEGF的蛋白表达均显著降低(P值均<0.05);与鳖甲软肝组相比,抗纤抑癌组mTOR的蛋白表达较高(P<0.01)。 结论 抗纤抑癌方可通过调控mTOR/HIF-1α/VEGF信号抑制肝癌前病变。 -
关键词:
- 肝肿瘤 /
- 信号传导 /
- 抗纤抑癌方 /
- 大鼠, Wistar
Abstract:Objective To investigate the effect of Kangxian Yiai Prescription (KXYA) on the mTOR/HIF-1α/VEGF signaling pathway in a rat model of precancerous lesions of liver cancer. Methods A total of 40 male Wistar rats were divided into normal group, model group, KXYA group, and Biejia Rangan Tablets (BJRG) group, with 10 rats in each group. The rats in the normal group were given intraperitoneal injection of normal saline at a dose of 0.4 mL/100 g, and those in the other three groups were given intraperitoneal injection of diethylnitrosamine at a dose of 50 mg/kg to establish a rat model of the precancerous lesions of liver cancer. Immunohistochemistry and Western Blot were used to measure the expression level of GST-Pi, and quantitative real-time PCR and Western Blot were used to measure the mRNA and protein expression levels of mTOR, HIF-1α, VEGF, PKM2, and GLUT1. A one-way analysis of variance or the Kruskal-Wallis H test was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups. Results Compared with the normal group, the model group had a significant increase in the protein expression level of GST-Pi in liver tissue (P<0.01), and compared with the model group, the KXYA group had a significant reduction in the protein expression level of GST-Pi (P<0.05). Compared with the normal group, the model group had significant increases in the mRNA expression levels of GLUT1 and PKM2 in liver tissue (P<0.01), and compared with the model group, the BJRG group and the KXYA group had a significant reduction in the mRNA expression level of GLUT1 (P<0.05). Compared with the normal group, the model group had significant increases in the protein expression levels of GLUT1 and PKM2 in liver tissue (P<0.01). Compared with the normal group, the model group had significant increases in the mRNA expression levels of mTOR, HIF-1α, and VEGF in liver tissue (P<0.01); compared with the model group, the BJRG group had significant reductions in the mRNA expression levels of mTOR and VEGF (P<0.05), and the KXYA group also had significant reductions in the mRNA expression levels of mTOR and VEGF (P<0.01). Compared with the normal group, the model group had significant increases in the protein expression levels of mTOR, HIF-1α, and VEGF in liver tissue (P<0.01); compared with the model group, the BJRG group had a significant reduction in the protein expression level of mTOR (P<0.01), and the KXYA group had significant reductions in the protein expression levels of mTOR, HIF-1α, and VEGF (P<0.05); compared with the BJRG group, the KXYA group had a significantly higher protein expression level of mTOR (P<0.01). Conclusion KXYA can inhibit the precancerous lesions of liver cancer by regulating the mTOR/HIF-1α/VEGF signaling pathway. -
Key words:
- Liver Neoplasms /
- Signal Transduction /
- Kangxian-Yiai Formula /
- Rats, Wistar
-
表 1 实时荧光定量PCR引物序列
Table 1. Real time fluorescence quantitative PCR primer sequence
引物名称 引物序列(5'-3') 扩增产物长度(bp) Rat-mTOR F:TGTCAGCCTGTCAGAATCCA 74 R:CCATGTTGACCAGCATTTCA Rat-HIF-1α F:TGGAAGCACTAGACAAAGCTCA 78 R:TTGACCATATCGCTGTCCAC Rat-VEGF F:GAGTTAAACGAACGTACTTGCAGA 90 R:TCTAGTTCCCGAAACCCTGA Rat-PKM2 F:GGAGAAGTGCGATGAGAACAT 141 R:TCTGTCACCAGGTAGTCAGCAC Rat-GLUT1 F:GTATCCTGTTGCCCTTCTGC 95 R:TCGAAGCTTTTTCAGCACAC GAPDH F:TCATTGACCTCAACTACATGG 131 R:TCGCTCCTGGAAGATGGTG -
[1] VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380( 15): 1450- 1462. DOI: 10.1056/nejmra1713263. [2] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [3] Chinese Journal of Hepatology; Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association. Expert consensus on multidisciplinary diagnosis and treatment of precancerous lesions of hepatocellular carcinoma(2020 edition)[J]. J Clin Hepatol, 2020, 36( 3): 514- 518. DOI: 10.3969/j.issn.1001-5256.2020.03.007.《中华肝脏病杂志》编辑委员会, 中华医学会肝病学分会肝癌学组. 肝细胞癌癌前病变的诊断和治疗多学科专家共识(2020版)[J]. 临床肝胆病杂志, 2020, 36( 3): 514- 518. DOI: 10.3969/j.issn.1001-5256.2020.03.007. [4] LIU H, CHEN XT, WANG PF, et al. PRMT1-mediated PGK1 arginine methylation promotes colorectal cancer glycolysis and tumorigenesis[J]. Cell Death Dis, 2024, 15( 2): 170. DOI: 10.1038/s41419-024-06544-6. [5] LI ZG, YANG XZ, LI XK, et al. Association of hypoxic microenvironment with the development and progression of liver diseases[J]. J Clin Hepatol, 2020, 36( 8): 1891- 1895. DOI: 10.3969/j.issn.1001-5256.2020.08.047.李志国, 杨先照, 李小科, 等. 缺氧微环境与肝病发生发展的关系[J]. 临床肝胆病杂志, 2020, 36( 8): 1891- 1895. DOI: 10.3969/j.issn.1001-5256.2020.08.047. [6] LAPLANTE M, SABATINI DM. mTOR signaling in growth control and disease[J]. Cell, 2012, 149( 2): 274- 293. DOI: 10.1016/j.cell.2012.03.017. [7] SAXTON RA, SABATINI DM. mTOR signaling in growth, metabolism, and disease[J]. Cell, 2017, 169( 2): 361- 371. DOI: 10.1016/j.cell.2017.03.035. [8] YANG XG, LU Y, HANG JJ, et al. Lactate-modulated immunosuppression of myeloid-derived suppressor cells contributes to the radioresistance of pancreatic cancer[J]. Cancer Immunol Res, 2020, 8( 11): 1440- 1451. DOI: 10.1158/2326-6066.CIR-20-0111. [9] MUSLEH UD DIN S, STREIT SG, HUYNH BT, et al. Therapeutic targeting of hypoxia-inducible factors in cancer[J]. Int J Mol Sci, 2024, 25( 4): 2060. DOI: 10.3390/ijms25042060. [10] SCHITO L, SEMENZA GL. Hypoxia-inducible factors: Master regulators of cancer progression[J]. Trends Cancer, 2016, 2( 12): 758- 770. DOI: 10.1016/j.trecan.2016.10.016. [11] GIANNITRAPANI L, DI GAUDIO F, CERVELLO M, et al. Genetic biomarkers of sorafenib response in patients with hepatocellular carcinoma[J]. Int J Mol Sci, 2024, 25( 4): 2197. DOI: 10.3390/ijms25042197. [12] FAIVRE S, RIMASSA L, FINN RS. Molecular therapies for HCC: Looking outside the box[J]. J Hepatol, 2020, 72( 2): 342- 352. DOI: 10.1016/j.jhep.2019.09.010. [13] LIU RJ, YANG XZ, ZHANG P, et al. Efficacy observation on Kangxian yi’ai formula in rats with liver precancerous lesions[J]. World Chin Med, 2015, 10( 9): 1309- 1312. DOI: 10.3969/j.issn.1673-7202.2015.09.004.刘蕊洁, 杨先照, 张鹏, 等. 抗纤抑癌方干预大鼠肝癌前病变疗效观察[J]. 世界中医药, 2015, 10( 9): 1309- 1312. DOI: 10.3969/j.issn.1673-7202.2015.09.004. [14] LI Y, YE YA, LI ZG, et al. Study on the mechanism of kangxianyiai formula delay the occurrence of hepatic precancerous lesions by regulating the signal pathway of PI3K-akt[J]. Chin J Integr Tradit West Med Liver Dis, 2019, 29( 3): 240- 243, 289. DOI: 10.3969/j.issn.1005-0264.2019.03.014.李莹, 叶永安, 李志国, 等. 基于PI3K/Akt信号通路探讨抗纤抑癌方干预肝癌前病变的作用机制[J]. 中西医结合肝病杂志, 2019, 29( 3): 240- 243, 289. DOI: 10.3969/j.issn.1005-0264.2019.03.014. [15] LIU LP, HO RL, CHEN GG, et al. Sorafenib inhibits hypoxia-inducible factor-1α synthesis: Implications for antiangiogenic activity in hepatocellular carcinoma[J]. Clin Cancer Res, 2012, 18( 20): 5662- 5671. DOI: 10.1158/1078-0432.CCR-12-0552. [16] WANG XB, WANG N, CHEUNG F, et al. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: Current progress on pharmacological actions and mechanisms[J]. J Integr Med, 2015, 13( 3): 142- 164. DOI: 10.1016/S2095-4964(15)60171-6. [17] HU B, AN HM, WANG SS, et al. Preventive and therapeutic effects of Chinese herbal compounds against hepatocellular carcinoma[J]. Molecules, 2016, 21( 2): 142. DOI: 10.3390/molecules21020142. [18] XU F, ZENG YL, LI J, et al. Mechanism of traditional Chinese medicine compound in preventing and treating hepatocellular carcinoma[J]. Chin J Exp Tradit Med Formulae, 2019, 25( 24): 196- 204. DOI: 10.13422/j.cnki.syfjx.20191921.徐菲, 曾杨丽, 李娟, 等. 中药复方防治肝癌作用机制研究进展[J]. 中国实验方剂学杂志, 2019, 25( 24): 196- 204. DOI: 10.13422/j.cnki.syfjx.20191921. [19] LI Y, YE YA, ZHANG LD, et al. Effects of Kangxian Yi’ai Formula on integrin α5β1/FAK signaling pathway in rats with hepatic precancerous lesions[J]. China J Tradit Chin Med Pharm, 2019, 34( 2): 759- 762.李莹, 叶永安, 张露丹, 等. 抗纤抑癌方对肝癌前病变大鼠整合素α5β1/FAK信号通路的影响[J]. 中华中医药杂志, 2019, 34( 2): 759- 762. [20] PAKRAVAN K, BABASHAH S, SADEGHIZADEH M, et al. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells[J]. Cell Oncol, 2017, 40( 5): 457- 470. DOI: 10.1007/s13402-017-0335-7. [21] MIYAZAWA M, YASUDA M, FUJITA M, et al. Therapeutic strategy targeting the mTOR-HIF-1alpha-VEGF pathway in ovarian clear cell adenocarcinoma[J]. Pathol Int, 2009, 59( 1): 19- 27. DOI: 10.1111/j.1440-1827.2008.02320.x. [22] WAN XL, SHEN N, MENDOZA A, et al. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling[J]. Neoplasia, 2006, 8( 5): 394- 401. DOI: 10.1593/neo.05820. -



 PDF下载 ( 1845 KB)
PDF下载 ( 1845 KB)

 下载:
下载: