血浆胆汁酸在肝肿瘤中的差异分析及应用价值
DOI: 10.12449/JCH241018
-
摘要:
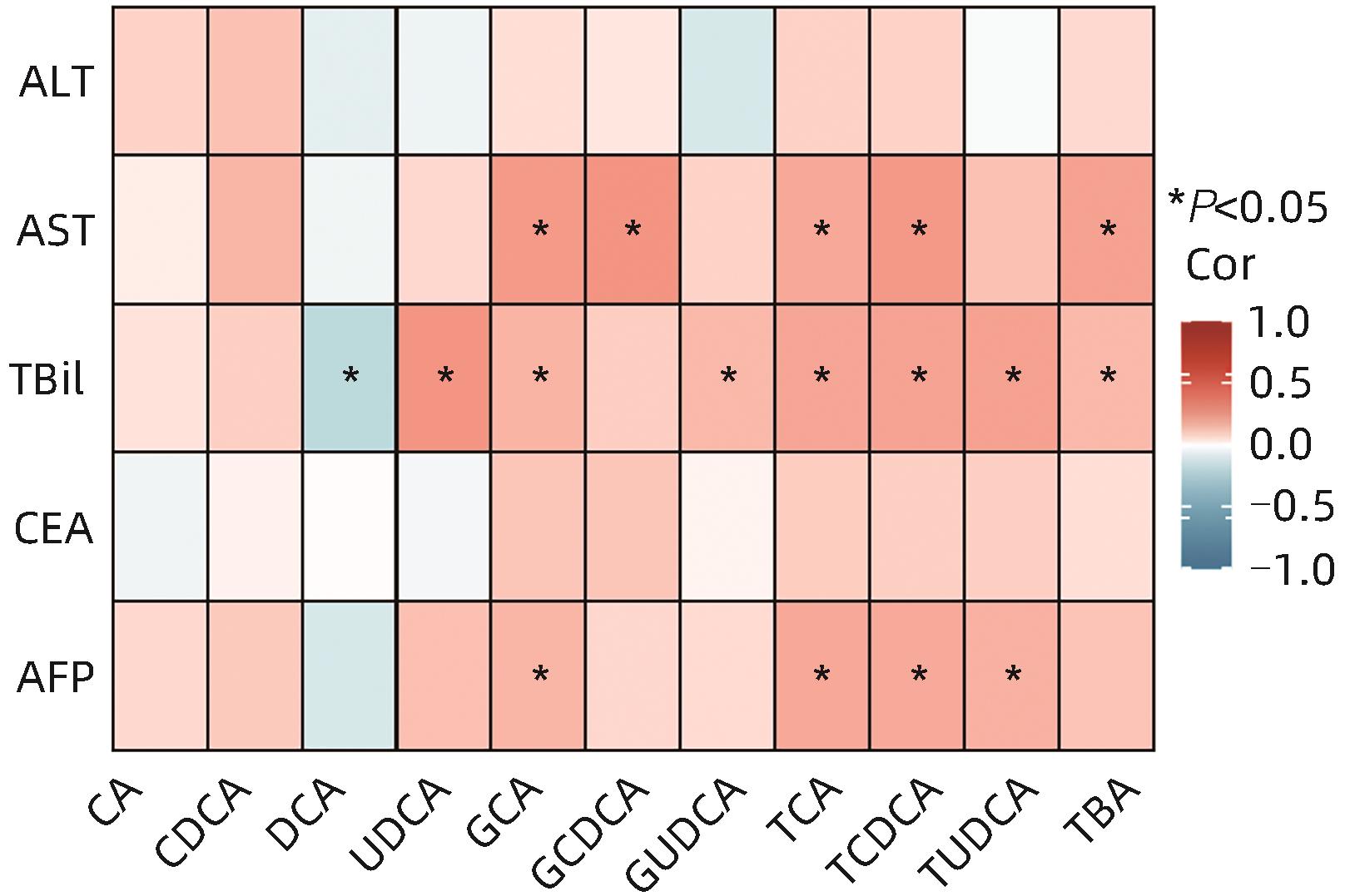
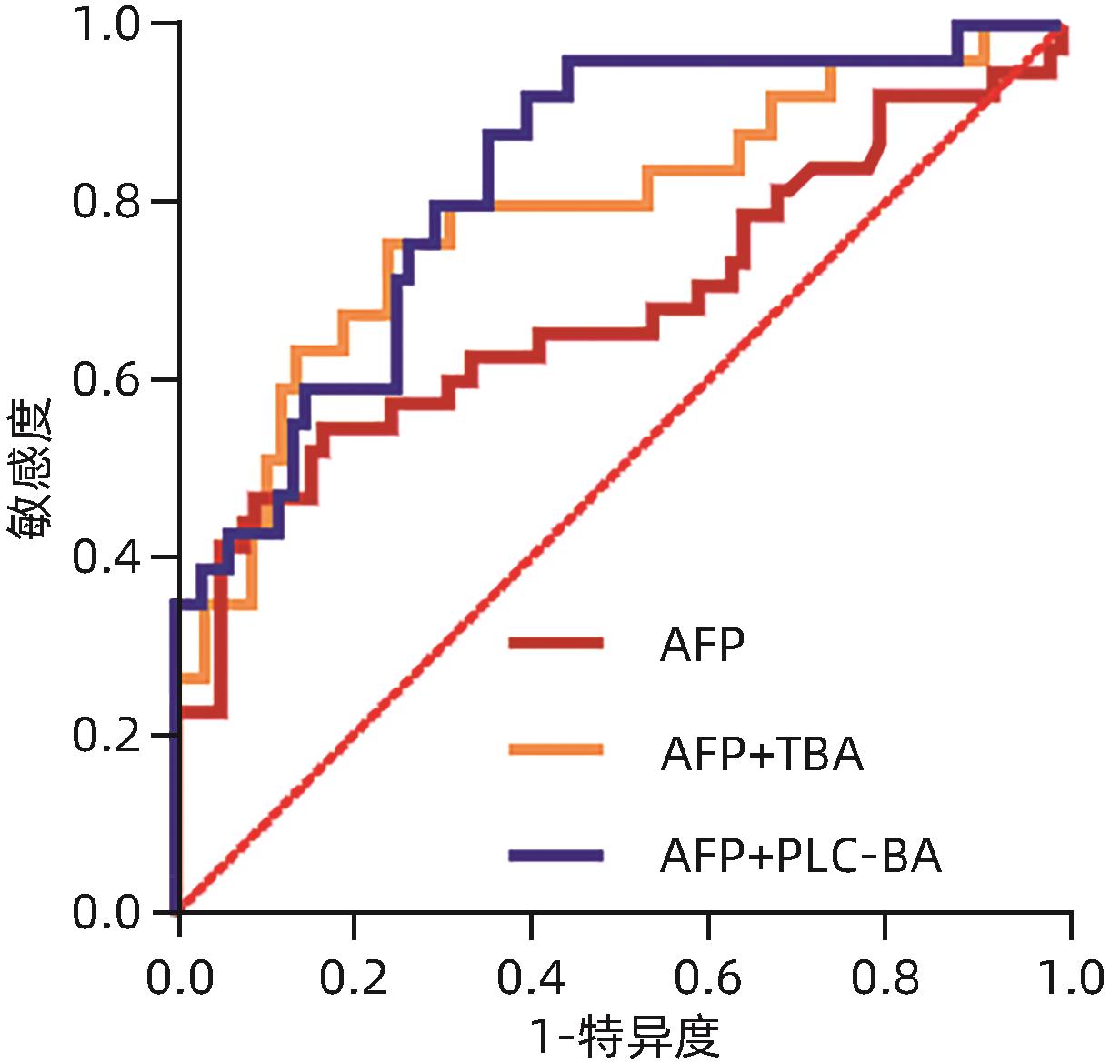
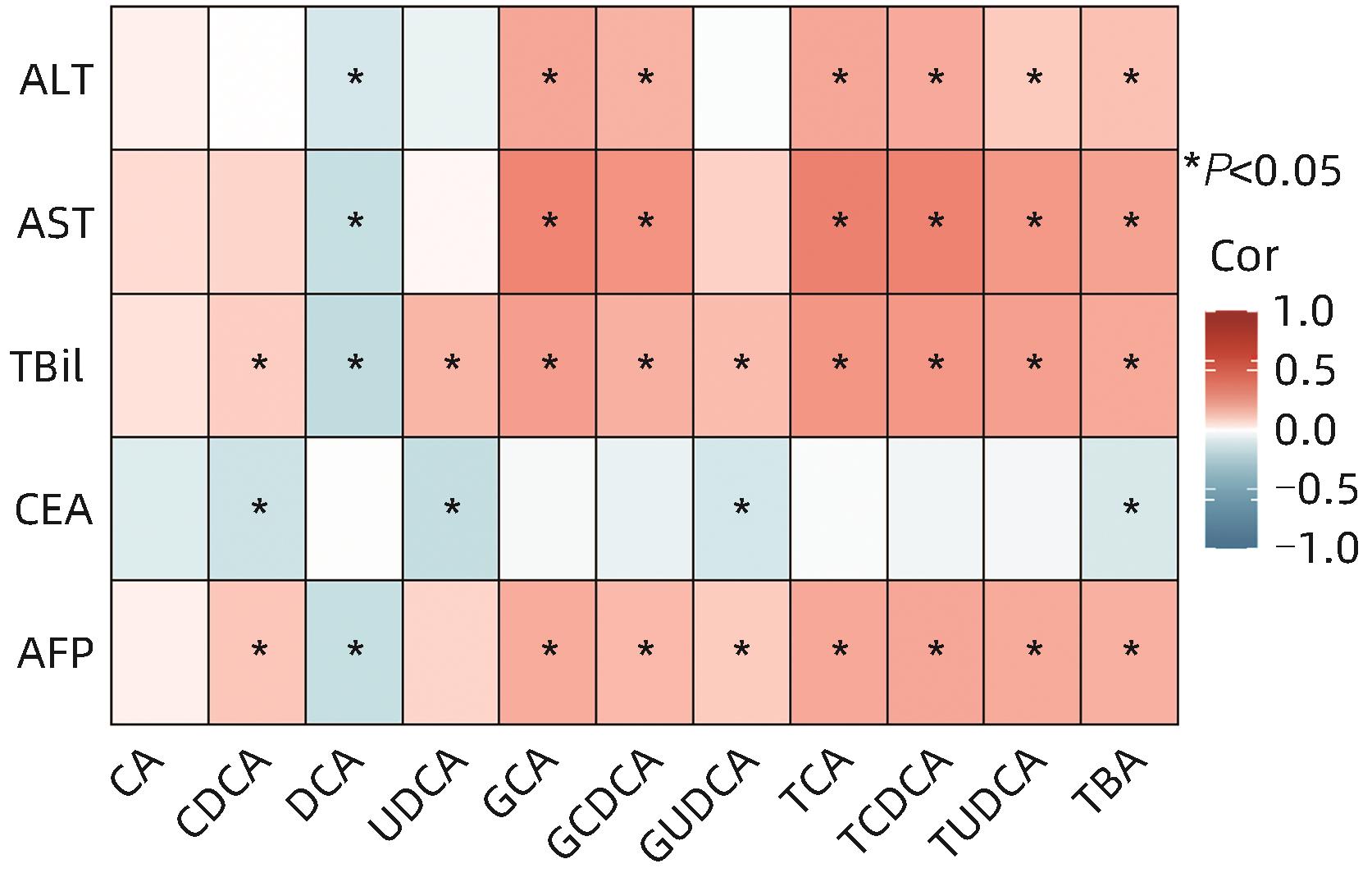
目的 分析原发性肝癌与转移性肝癌患者血浆胆汁酸水平及其与临床指标的相关性,并评估其联合AFP对于原发性肝癌的诊断价值。 方法 纳入2020年8月—2021年9月于上海中医药大学附属曙光医院就诊且具有组织病理学及影像学明确的75例原发性肝癌患者及79例转移性肝癌患者,采集外周血并分别分离血清及血浆,比色法及色谱法检测生化指标,电化学发光免疫分析法检测肿瘤标志物水平,高效液相色谱-串联质谱法检测胆汁酸含量。符合正态分布的计量资料两组间比较采用成组t检验,不符合正态分布的计量资料两组间比较采用Mann-Whitney U检验。相关性检验采用Spearman相关分析。临床诊断效能采用受试者工作特征曲线(ROC曲线)评估。 结果 原发性肝癌组患者TC、TG、LDL-C及载脂蛋白(Apo)B显著低于转移性肝癌组患者,差异均具有统计学意义(U值分别为1 598、1 255、909、889,P值均<0.05)。原发性肝癌组患者AFP显著高于转移性肝癌组患者,癌胚抗原(CEA)显著低于转移性肝癌组患者,差异均具有统计学意义(U值分别为1 873、926,P值均<0.05)。原发性肝癌组患者总胆汁酸(TBA)、胆酸(CA)、鹅脱氧胆酸(CDCA)、熊脱氧胆酸(UDCA)、牛磺胆酸(TCA)、牛磺鹅脱氧胆酸(TCDCA)、甘氨胆酸(GCA)、甘氨鹅脱氧胆酸(GCDCA)、牛磺熊脱氧胆酸(TUDCA)、甘氨熊脱氧胆酸(GUDCA)均显著高于转移性肝癌患者,脱氧胆酸(DCA)显著低于转移性肝癌患者,差异均具有统计学意义(P值均<0.05)。总人群中TBA、CDCA、GCA、GCDCA、GUDCA、TCA、TCDCA及TUDCA含量与AFP水平呈显著正相关(P值均<0.05)。原发性肝癌患者中GCA、TCA、TCDCA及TUDCA含量与AFP水平呈显著正相关(P值均<0.05)。AFP+TCA+GCA+TCDCA联合检测的AUC为0.822(95%CI:0.746~0.898,P<0.000 1),效能最高。 结论 原发性肝癌与转移性肝癌患者血浆胆汁酸含量具有显著差异,差异性胆汁酸与肝损伤及AFP升高密切相关,联合AFP对原发性肝癌具有更优的临床诊断价值。 Abstract:Objective To investigate the levels of plasma bile acids (BA) in patients with primary liver cancer (PLC) or metastatic liver cancer (MLC) and their correlation with clinical indicators, as well as the value of plasma BAs combined with alpha-fetoprotein (AFP) in the diagnosis of PLC. Methods This study was conducted among 75 patients with PLC and 79 patients with MLC who attended Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine from August 2020 to September 2021 and had a confirmed diagnosis based on histopathological and imaging findings. Peripheral blood samples were collected from all patients, and serum and plasma were separated. Colorimetry and chromatography were used to measure biochemical parameters; electrochemiluminescence immunoassay was used to measure the levels of tumor markers; liquid chromatography-tandem mass spectrometry was used to measure the content of BA. The t-test was used for comparison of normally distributed continuous data, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data; the Spearman’s coefficient was used for correlation analysis; the receiver operating characteristic (ROC) curve was used to evaluate clinical diagnostic efficacy. Results The PLC group had significantly lower levels of total cholesterol, triglyceride, low-density lipoprotein cholesterol, and apolipoprotein B than the MLC group (U=1 598, 1 255, 909, and 889, all P<0.05). Compared with the MLC group, the PLC group had a significantly higher level of AFP and a significantly lower level of carcinoembryonic antigen (U=1 873 and 926, both P<0.05). Compared with the MLC group, the PLC group had significantly higher levels of TBA, CA, CDCA, UDCA, TCA, TCDCA, GCA, GCDCA, TUDCA, and GUDCA and a significantly lower level of DCA (all P<0.05). In the total population, the levels of TBA, CDCA, GCA, GCDCA, GUDCA, TCA, TCDCA, and TUDCA were significantly positively correlated with the level of AFP (all P<0.05). In the patients with PLC, the levels of GCA, TCA, TCDCA, and TUDCA were significantly positively correlated with the level of AFP (all P<0.05). Combined measurement of AFP+TCA+GCA+TCDCA had an area under the ROC curve of 0.822 (95% confidence interval: 0.746 — 0.898, P<0.000 1), suggesting that it had the highest diagnostic efficacy. Conclusion There are significant differences in the levels of plasma BA between the patients with PLC and those with MLC, and the differentially expressed BAs are closely associated with liver function impairment and the increase in AFP. BAs combined with AFP has a better clinical value in the diagnosis of PLC. -
Key words:
- Bile Acids and Salts /
- Liver Neoplasms /
- Neoplasm Metastasis
-
表 1 两组患者生化指标比较
Table 1. Comparison of levels of laboratory tests between patients with primary liver cancer or metastatic liver cancer
项目 原发性肝癌组(n=75) 转移性肝癌组(n=79) 统计值 P值 ALT(U/L) 25.00(18.00~33.00) 22.00(12.00~37.00) U=2 375 0.177 AST(U/L) 31.00(27.25~52.00) 29.00(20.75~46.00) U=1 933 0.063 TBil(μmol/L) 20.10(13.70~30.90) 15.85(12.63~28.80) U=2 407 0.059 GLU(mmol/L) 5.30(4.60~6.00) 5.05(4.70~6.00) U=2 636 0.925 HbA1c(%) 5.40(4.90~6.00) 5.50(5.10~6.00) U=2 140 0.205 TC(mmol/L) 3.87(3.31~4.58) 4.81(4.18~5.91) U=1 598 <0.001 TG(mmol/L) 1.04(0.88~1.38) 1.31(1.07~1.85) U=1 255 <0.001 HDL-C(mmol/L) 1.07±0.29 1.09±0.34 t=0.243 0.867 LDL-C(mmol/L) 2.09(1.45~2.73) 3.06(2.38~4.08) U=909 <0.001 ApoA1(g/L) 0.90(0.73~1.06) 0.93(0.81~1.14) U=1 474 0.154 ApoB(g/L) 0.67(0.49~0.89) 0.93(0.69~1.16) U=889 <0.001 表 2 两组患者肿瘤标志物水平比较
Table 2. Comparison of levels of serum tumor markers between patients with primary liver cancer or metastatic liver cancer
项目 原发性肝癌组(n=75) 转移性肝癌组(n=79) U值 P值 AFP(ng/mL) 6.45(3.16~15.74) 4.02(2.54~5.23) 1 873 <0.001 CEA(ng/mL) 3.04(2.53~5.42) 15.14(5.02~83.18) 926 <0.001 CA19-9(U/mL) 21.20(10.83~54.69) 21.36(10.22~154.38) 2 361 0.840 CA125(U/mL) 18.70(12.50~36.20) 21.20(10.55~44.63) 1 181 0.128 表 3 两组患者血浆胆汁酸含量比较
Table 3. Comparison of concentrations of plasma BA between patients with primary liver cancer or metastatic liver cancer
项目 原发性肝癌组(n=75) 转移性肝癌组(n=79) U值 P值 TBA(nmol/L) 11 675.7(6 098.5~33 679.9) 5 241.6(2 445.8~10 062.8) 886 <0.001 CA(nmol/L) 290.7(148.6~1 030.0) 114.0(62.8~319.0) 1 084 <0.001 CDCA(nmol/L) 2 570.0(766.4~3 040.0) 527.0(191.5~1 127.2) 750 <0.001 DCA(nmol/L) 66.9(6.9~354.0) 238.3(19.1~572.0) 1 223 0.010 UDCA(nmol/L) 199.0(109.5~572.5) 68.7(26.8~246.5) 1 039 <0.001 LCA(nmol/L) 15.7(6.3~45.6) 21.2(10.7~39.9) 1 476 0.243 TCA(nmol/L) 444.0(90.3~2 231.1) 47.2(11.3~482.2) 862 <0.001 TCDCA(nmol/L) 627.0(256.0~5 060.0) 145.0(48.7~981.9) 828 <0.001 GCA(nmol/L) 1 309.2(424.0~4 670.0) 444.6(190.5~1 400.4) 1 018 <0.001 GCDCA(nmol/L) 3 530.0(1 160.0~13 341.0) 1 149.3(388.4~3 537.4) 978 <0.001 TDCA(nmol/L) 23.6(0.0~114.0) 25.6(5.0~92.3) 1 423 0.144 TUDCA(nmol/L) 41.8(10.8~117.0) 7.3(1.9~21.5) 832 <0.001 TLCA(nmol/L) 0.9(0.2~4.0) 0.9(0.0~2.3) 1 520 0.352 GDCA(nmol/L) 119.8(5.9~609.0) 187.0(13.7~498.0) 1 586 0.570 GUDCA(nmol/L) 434.0(156.8~1 390.0) 104.0(49.9~637.1) 986 <0.001 GLCA(nmol/L) 5.2(0.6~29.0) 6.4(1.8~19.8) 1 641 0.789 -
[1] JIA W, XIE GX, JIA WP. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis[J]. Nat Rev Gastroenterol Hepatol, 2018, 15( 2): 111- 128. DOI: 10.1038/nrgastro.2017.119. [2] CHÁVEZ-TALAVERA O, TAILLEUX A, LEFEBVRE P, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease[J]. Gastroenterology, 2017, 152( 7): 1679- 1694.e3. DOI: 10.1053/j.gastro.2017.01.055. [3] SMIRNOVA E, MUTHIAH MD, NARAYAN N, et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD[J]. Hepatology, 2022, 76( 6): 1811- 1824. DOI: 10.1002/hep.32568. [4] CHEN T, WANG L, XIE G, et al. Serum bile acids improve prediction of Alzheimer's progression in a sex-dependent manner[J]. Adv Sci(Weinh), 2024, 11( 9): e2306576. DOI: 10.1002/advs.202306576. [5] SONG WS, PARK HM, HA JM, et al. Discovery of glycocholic acid and taurochenodeoxycholic acid as phenotypic biomarkers in cholangiocarcinoma[J]. Sci Rep, 2018, 8( 1): 11088. DOI: 10.1038/s41598-018-29445-z. [6] JI SY, LIU QX, ZHANG SH, et al. FGF15 activates hippo signaling to suppress bile acid metabolism and liver tumorigenesis[J]. Dev Cell, 2019, 48( 4): 460- 474. e 9. DOI: 10.1016/j.devcel.2018.12.021. [7] SUN RQ, ZHANG ZY, BAO RX, et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis[J]. J Hepatol, 2022, 77( 2): 453- 466. DOI: 10.1016/j.jhep.2022.02.030. [8] LIU ZR, JIA XD, LU YY. Research advances in hepatocellular carcinoma-related imbalance of bile acid metabolism and related regulatory mechanism[J]. J Clin Hepatol, 2021, 37( 3): 690- 694. DOI: 10.3969/j.issn.1001-5256.2021.03.038.刘哲睿, 贾晓东, 陆荫英. 肝细胞癌相关胆汁酸代谢失衡及调控机制的研究进展[J]. 临床肝胆病杂志, 2021, 37( 3): 690- 694. DOI: 10.3969/j.issn.1001-5256.2021.03.038. [9] SYDOR S, BEST J, MESSERSCHMIDT I, et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC[J]. Clin Transl Gastroenterol, 2020, 11( 3): e00131. DOI: 10.14309/ctg.0000000000000131. [10] PETRICK JL, FLORIO AA, KOSHIOL J, et al. Prediagnostic concentrations of circulating bile acids and hepatocellular carcinoma risk: REVEAL-HBV and HCV studies[J]. Int J Cancer, 2020, 147( 10): 2743- 2753. DOI: 10.1002/ijc.33051. [11] LI YY, LI JJ, SHI HF, et al. Advances in imaging research on source identification of primary liver metastases[J]. J Clin Radiol, 2023, 42( 5): 878- 881. DOI: 10.13437/j.cnki.jcr.2023.05.007.黎玉莹, 李晶晶, 石海峰, 等. 肝转移瘤原发灶来源鉴别的影像学研究进展[J]. 临床放射学杂志, 2023, 42( 5): 878- 881. DOI: 10.13437/j.cnki.jcr.2023.05.007. [12] JIA W, WEI ML, RAJANI C, et al. Targeting the alternative bile acid synthetic pathway for metabolic diseases[J]. Protein Cell, 2021, 12( 5): 411- 425. DOI: 10.1007/s13238-020-00804-9. [13] LI TG, CHIANG JYL. Bile acid signaling in metabolic disease and drug therapy[J]. Pharmacol Rev, 2014, 66( 4): 948- 983. DOI: 10.1124/pr.113.008201. [14] WANG CZ, YANG MY, ZHAO JF, et al. Bile salt(glycochenodeoxycholate acid) induces cell survival and chemoresistance in hepatocellular carcinoma[J]. J Cell Physiol, 2019, 234( 7): 10899- 10906. DOI: 10.1002/jcp.27905. [15] MA C, HAN MJ, HEINRICH B, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells[J]. Science, 2018, 360( 6391): eaan5931. DOI: 10.1126/science.aan5931. [16] SARKAR J, AOKI H, WU RR, et al. Conjugated bile acids accelerate progression of pancreatic cancer metastasis via S1PR2 signaling in cholestasis[J]. Ann Surg Oncol, 2023, 30( 3): 1630- 1641. DOI: 10.1245/s10434-022-12806-4. [17] CHENG P, WU JW, ZONG GF, et al. Capsaicin shapes gut microbiota and pre-metastatic niche to facilitate cancer metastasis to liver[J]. Pharmacol Res, 2023, 188: 106643. DOI: 10.1016/j.phrs.2022.106643. [18] HAN J, QIN WX, LI ZL, et al. Tissue and serum metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma[J]. Clin Chim Acta, 2019, 488: 68- 75. DOI: 10.1016/j.cca.2018.10.039. [19] LUO P, YIN PY, HUA R, et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma[J]. Hepatology, 2018, 67( 2): 662- 675. DOI: 10.1002/hep.29561. [20] XIAN LF, FANG LT, LIU WB, et al. Epidemiological status, main pathogenesis and prevention and control strategies of primary liver cancer[J]. Chin J Oncol Prev Treat, 2022, 14( 3): 320- 328. DOI: 10.3969/j.issn.1674-5671.2022.03.13.鲜林峰, 方乐天, 刘文斌, 等. 原发性肝癌流行现状、主要发病机制及防控策略[J]. 中国癌症防治杂志, 2022, 14( 3): 320- 328. DOI: 10.3969/j.issn.1674-5671.2022.03.13. [21] The Society of Liver Cancer, China Anti-Cancer Association. CACA guidelines for holistic integrative management of cancer-liver cancerpart[J/CD]. J Multidiscip Cancer Manag Electron Version, 2022, 8( 3): 31- 63. DOI: 10.12151/JMCM.2022.03-06.中国抗癌协会肝癌专业委员会. 中国肿瘤整合诊治指南(CACA)-肝癌部分[J/CD]. 肿瘤综合治疗电子杂志, 2022, 8( 3): 31- 63. DOI: 10.12151/JMCM.2022.03-06. [22] TIAN CX, ZHAO L. Epidemiological characteristics of colorectal cancer and colorectal liver metastasis[J]. Chin J Cancer Prev Treat, 2021, 28( 13): 1033- 1038. DOI: 10.16073/j.cnki.cjcpt.2021.13.12.田传鑫, 赵磊. 结直肠癌及结直肠癌肝转移流行病学特点[J]. 中华肿瘤防治杂志, 2021, 28( 13): 1033- 1038. DOI: 10.16073/j.cnki.cjcpt.2021.13.12. [23] WANG ZJ, KIM SY, TU W, et al. Extracellular vesicles in fatty liver promote a metastatic tumor microenvironment[J]. Cell Metab, 2023, 35( 7): 1209- 1226. e 13. DOI: 10.1016/j.cmet.2023.04.013. [24] XIAO JF, VARGHESE RS, ZHOU B, et al. LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort[J]. J Proteome Res, 2012, 11( 12): 5914- 5923. DOI: 10.1021/pr300673x. [25] ZENG HW, UMAR S, RUST B, et al. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer[J]. Int J Mol Sci, 2019, 20( 5): 1214. DOI: 10.3390/ijms20051214. [26] LI JY, GILLILLAND M 3rd, LEE AA, et al. Secondary bile acids mediate high-fat diet-induced upregulation of R-spondin 3 and intestinal epithelial proliferation[J]. JCI Insight, 2022, 7( 19): e148309. DOI: 10.1172/jci.insight.148309. [27] CHEN TL, XIE GX, WANG XY, et al. Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma[J]. Mol Cell Proteomics, 2011, 10( 7): M110.004945. DOI: 10.1074/mcp.M110.004945. [28] COLLINS SL, STINE JG, BISANZ JE, et al. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease[J]. Nat Rev Microbiol, 2023, 21( 4): 236- 247. DOI: 10.1038/s41579-022-00805-x. [29] ZHANG YF, LI MC. Application value of chitosanase 3-like protein 1, alpha-fetoprotein, glycoactrin 19-9, glutamyltranspeptidase in the early diagnosis of primary liver cancer[J]. Chin J Health Lab Technol, 2023, 33( 4): 467- 470.张艳芬, 李明才. 血清壳多糖酶3样蛋白1、甲胎蛋白、糖类抗原19-9及谷氨酰转肽酶在原发性肝癌早期诊断中的应用价值[J]. 中国卫生检验杂志, 2023, 33( 4): 467- 470. [30] YANG YP, XU EJ, WANG XX, et al. The value of GNB4 and Riplet gene methylation detection in the diagnosis of primary liver cancer[J]. Acta Univ Med Anhui, 2024, 59( 2): 357- 362. DOI: 10.19405/j.cnki.issn1000-1492.2024.02.028.杨玉萍, 徐恩君, 汪轩轩, 等. GNB4和Riplet基因甲基化检测在原发性肝癌诊断中的价值研究[J]. 安徽医科大学学报, 2024, 59( 2): 357- 362. DOI: 10.19405/j.cnki.issn1000-1492.2024.02.028. [31] ZHANG JL, SHEN Q, WANG N, et al. Values of serum 7 microRNAs alone or in combination with α-fetoprotein in diagnosing hepatocellular carcinoma[J]. Acad J Nav Med Univ, 2023, 44( 5): 636- 639. DOI: 10.16781/j.cn31-2187/r.20220648.张敬磊, 沈强, 王能, 等. 血清7种微RNA单独或联合甲胎蛋白检测对肝细胞癌的诊断价值[J]. 海军军医大学学报, 2023, 44( 5): 636- 639. DOI: 10.16781/j.cn31-2187/r.20220648. -



 PDF下载 ( 1078 KB)
PDF下载 ( 1078 KB)

 下载:
下载: