异常凝血酶原对肝细胞癌的诊断效能及其与肿瘤临床特征的相关性分析
DOI: 10.12449/JCH241014
Efficacy of des-γ-carboxy-prothrombin in the diagnosis of hepatocellular carcinoma and its association with the clinical features of hepatocellular carcinoma
-
摘要:
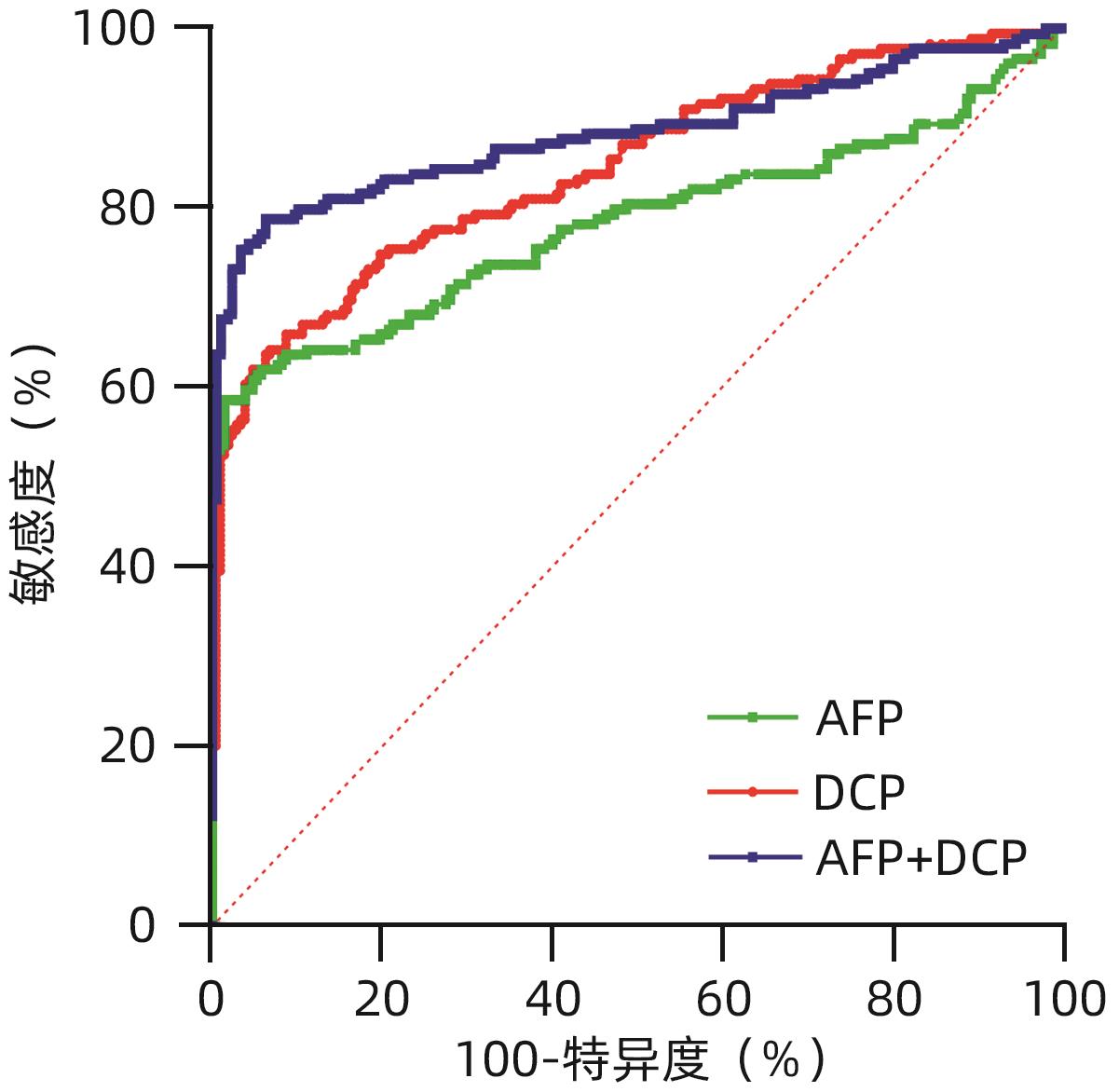
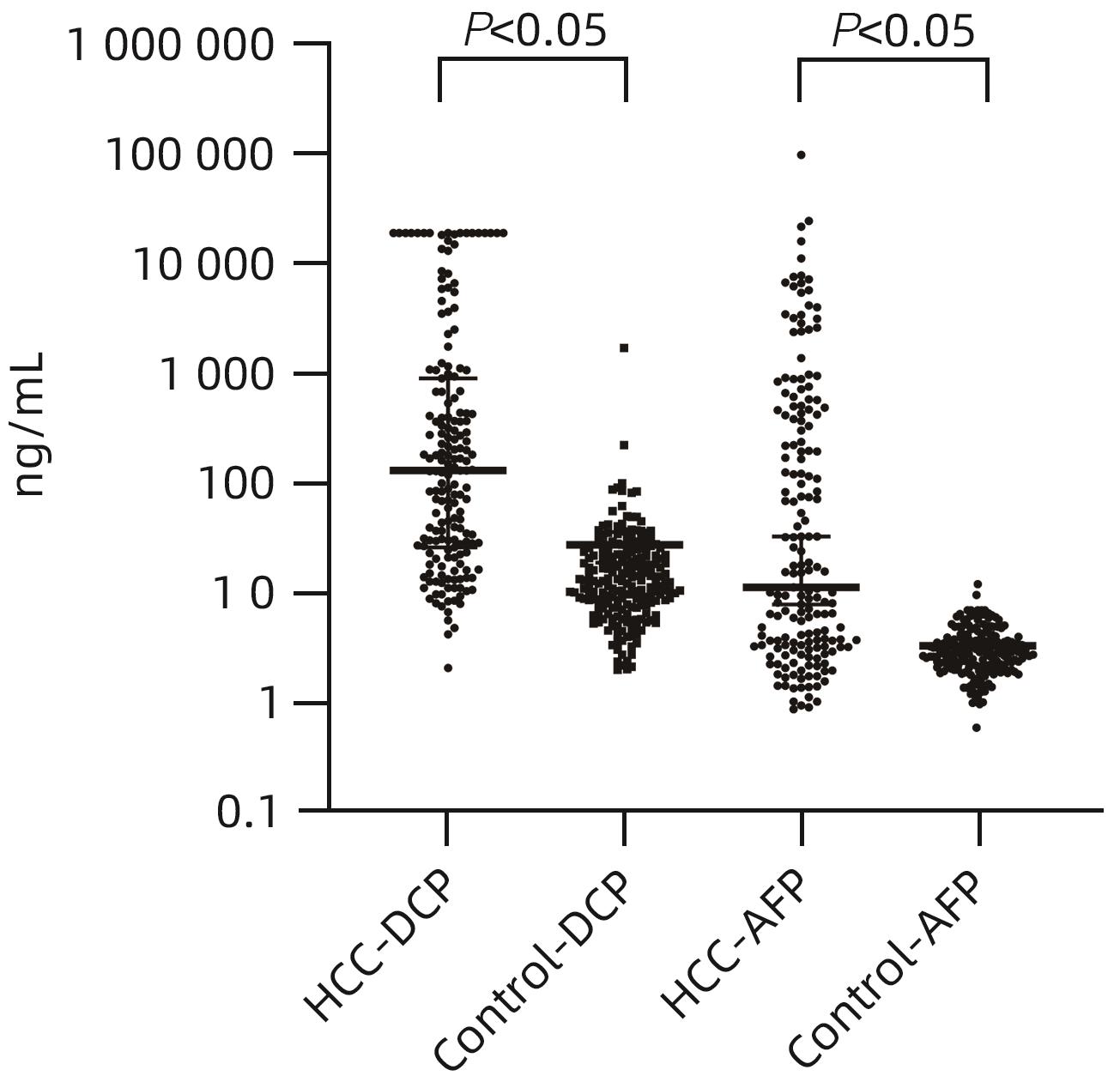
目的 评估异常凝血酶原(DCP)在肝细胞癌(HCC)临床应用中的价值。 方法 回顾性分析2020年1月—2021年7月吉林大学第一医院收治的179例HCC患者的临床资料,另纳入207例健康对照者。应用磁微粒化学发光免疫分析法测定AFP及DCP血清学水平。绘制单独及联合检测时的受试者工作特征曲线(ROC曲线),并计算曲线下面积(AUC),探究联合应用DCP及AFP相较于单独检测AFP对HCC的临床诊断价值,以及DCP对AFP阴性患者的诊断效能。不符合正态分布的计量资料组间比较采用Mann-Whitney U检验。通过ROC曲线分析评估诊断效率。通过Spearman相关性分析明确肿瘤标志物与HCC病理学特征的相关性。 结果 HCC患者血清AFP和DCP水平较正常患者显著升高(Z值分别为-9.562、-11.678,P值均<0.05),DCP和AFP的联合检测优于AFP单独检测(Z=5.309,P<0.01)。DCP存在对AFP阴性HCC患者的诊断能力(AUC=0.789,P<0.000 1),敏感度和特异度分别为61.64%和86.47%。血清DCP水平与肿瘤大小(r=0.546,P<0.001)、TNM分期(r=0.306,P<0.001)、微血管侵犯(r=0.358,P<0.001)呈正相关,与肿瘤分化程度呈负相关(r=-0.220,P<0.05)。 结论 AFP、DCP的联合检测可提高HCC检出率且DCP可用于AFP阴性HCC患者的补充筛查;DCP的表达水平与HCC临床病理特征(包括肿瘤大小、TNM分期、微血管侵犯、肿瘤分化程度)之间存在相关性。 Abstract:Objective To investigate the value of des-γ-carboxy-prothrombin (DCP) in hepatocellular carcinoma (HCC). Methods A retrospective analysis was performed for the clinical data of 179 HCC patients who were admitted to The First Hospital of Jilin University from January 2020 to July 2021, and 207 healthy controls were enrolled as normal group. Magnetic particle chemiluminescence immunoassay was used to measure the serological levels of alpha-fetoprotein (AFP) and DCP. The receiver operating characteristic (ROC) curve was plotted for each indicator measured alone or in combination, and the area under the ROC curve (AUC) was calculated to investigate the value of DCP combined with AFP versus AFP alone in the diagnosis of HCC and the diagnostic efficacy of DCP in AFP-negative patients. The Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups; the ROC curve was used to evaluate diagnostic efficiency; the Spearman correlation analysis was used to investigate the correlation of tumor markers with the pathological features of HCC. Results The patients with HCC had significantly higher serum levels of AFP and DCP than the normal group (Z=-9.562 and -11.678, P<0.05), and combined measurement of DCP and AFP had a better value than AFP measured alone (Z=5.309, P<0.01). DCP had certain capability in the diagnosis of AFP-negative HCC patients, with an AUC of 0.789 (P<0.000 1), a sensitivity of 61.64%, and a specificity of 86.47%. Serum DCP level was positively correlated with tumor size (r=0.546, P<0.001), TNM stage (r=0.306, P<0.001), and microvascular invasion (r=0.358,P<0.001) and was negatively correlated with the degree of tumor differentiation (r=-0.220, P<0.05). Conclusions The combined measurement of AFP and DCP can improve the detection rate of HCC, and DCP can be used for supplementary screening in AFP-negative HCC patients. The expression level of DCP is correlated with the clinicopathological features of HCC, including tumor size, TNM stage, microvascular invasion, and the degree of tumor differentiation. -
Key words:
- Prothrombin /
- Carcinoma, Hepatocellular /
- alpha-Fetoproteins /
- Biomarkers, Tumor
-
表 1 AFP、DCP及联合诊断的效能评估
Table 1. Effectiveness evaluation of AFP, DCP and combined diagnosis
指标 AUC Cut-off值 敏感度(%) 特异度(%) Z值 P值 AFP 0.782(0.738~0.822) 7.28 58.66(51.34~65.62) 98.55(95.83~99.60) DCP 0.845(0.804~0.879) 44.75 64.25(56.99~70.90) 93.24(88.97~95.93) 2.079 0.037 61) AFP+DCP 0.884(0.847~0.914) 78.77 93.72 5.309 <0.000 12) 注:与AFP组相比较,1)P<0.05;2)P<0.01。
表 2 DCP与HCC临床特征相关性分析
Table 2. Correlation analysis between DCP and clinicopathological features of HCC
指标 r值 P值 年龄 -0.092 0.220 性别
AFP
0.001
0.264
0.991
<0.001
肿瘤大小 0.546 <0.001 肿瘤数目 -0.046 0.543 TNM分期 0.306 <0.001 分化程度 -0.220 0.005 微血管侵犯 0.358 <0.001 卫星灶 0.089 0.237 -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] SIEGEL RL, MILLER KD, JEMAL A. Cancer statistics, 2020[J]. CA A Cancer J Clinicians, 2020, 70( 1): 7- 30. DOI: 10.3322/caac.21590. [3] General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer(2022 edition)[J]. J Clin Hepatol, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009.国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 临床肝胆病杂志, 2022, 38( 2): 288- 303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. [4] HE J, CHEN WQ, SHEN HB, et al. China guideline for liver cancer screening(2022, Beijing)[J]. J Clin Hepatol, 2022, 38( 8): 1739- 1758, 1954- 1967. DOI: 10.3969/j.issn.1001-5256.2022.08.007.赫捷, 陈万青, 沈洪兵, 等. 中国人群肝癌筛查指南(2022, 北京)[J]. 临床肝胆病杂志, 2022, 38( 8): 1739- 1758, 1954- 1967. DOI: 10.3969/j.issn.1001-5256.2022.08.007. [5] LIEBMAN HA, FURIE BC, TONG MJ, et al. Des-gamma-carboxy(abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma[J]. N Engl J Med, 1984, 310( 22): 1427- 1431. DOI: 10.1056/NEJM198405313102204. [6] DING CM, HOU JF, TAO GW, et al. Early diagnosis and screening of hepatocellular carcinoma[J/OL]. Chin J Hepatic Surg(Electronic Edition), 2023, 12( 1): 22- 28. DOI: 10.3877/cma.j.issn.2095-3232.2023.01.005.丁成明, 侯嘉丰, 陶光伟, 等. 肝细胞癌早期诊断和筛查[J/OL]. 中华肝脏外科手术学电子杂志, 2023, 12( 1): 22- 28. DOI: 10.3877/cma.j.issn.2095-3232.2023.01.005. [7] YUAN LW, TANG W, ZHOU JP, et al. Quantitative measurement of des-γ-carboxy-prothrombin in cancerous and non-cancerous liver tissue and its role in hepatocellular carcinoma[J]. World Chin J Dig, 2006, 14( 1): 45- 49. DOI: 10.3969/j.issn.1009-3079.2006.01.009.袁联文, 唐伟, 周建平, 等. 肝癌组织中脱-γ-羧基凝血酶原的测定及意义[J]. 世界华人消化杂志, 2006, 14( 1): 45- 49. DOI: 10.3969/j.issn.1009-3079.2006.01.009. [8] SHIMADA M, YAMASHITA Y, HAMATSU T, et al. The role of des-gamma-carboxy prothrombin levels in hepatocellular carcinoma and liver tissues[J]. Cancer Lett, 2000, 159( 1): 87- 94. DOI: 10.1016/s0304-3835(00)00539-5. [9] YUEN MF, LAI CL. Serological markers of liver cancer[J]. Best Pract Res Clin Gastroenterol, 2005, 19( 1): 91- 99. DOI: 10.1016/j.bpg.2004.10.003. [10] FUJIYAMA S, MORISHITA T, SAGARA K, et al. Clinical evaluation of plasma abnormal prothrombin(PIVKA-II) in patients with hepatocellular carcinoma[J]. Hepato-gastroenterology, 1986, 33( 5): 201- 205. DOI: 10.1007/BF02560343. [11] UEHARA S, GOTOH K, HANDA H, et al. Distribution of the heterogeneity of des-γ-carboxyprothrombin in patients with hepatocellular carcinoma[J]. J Gastro And Hepatol, 2005, 20( 10): 1545- 1552. DOI: 10.1111/j.1440-1746.2005.03899.x. [12] SEKIYA C, KOHDA H, HASEBE C, et al. Characteristics of the PIVKA-II found in hepatocellular carcinoma, investigation using monoclonal antibodies MU-3 and 19B7[J]. Int Hepatol Commun, 1994, 2( 5): 277- 284. DOI: 10.1016/0928-4346(94)90063-9. [13] OHHIRA M, OHTAKE T, SAITO H, et al. Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma[J]. Alcohol Clin Exp Res, 1999, 23( 4 Suppl): 67S- 70S. DOI: 10.1111/j.1530-0277.1999.tb04537.x. [14] MURATA K, SUZUKI H, OKANO H, et al. Hypoxia-induced des-gamma-carboxy prothrombin production in hepatocellular carcinoma[J]. Int J Oncol, 2010, 36( 1): 161- 170. [15] SUZUKI H, MURATA K, GOTOH T, et al. Phenotype-dependent production of des-γ-carboxy prothrombin in hepatocellular carcinoma[J]. J Gastroenterol, 2011, 46( 10): 1219- 1229. DOI: 10.1007/s00535-011-0432-8. [16] HAO X, FAN R, HOU JL. Early warning and accurate screening for the high-risk population of hepatocellular carcinoma[J]. J Clin Hepatol, 2022, 38( 3): 499- 504. DOI: 10.3969/j.issn.1001-5256.2022.03.002.郝新, 樊蓉, 侯金林. 原发性肝癌高危人群的早期预警和精准筛查[J]. 临床肝胆病杂志, 2022, 38( 3): 499- 504. DOI: 10.3969/j.issn.1001-5256.2022.03.002. [17] BEST J, BECHMANN LP, SOWA JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis[J]. Clin Gastroenterol Hepatol, 2020, 18( 3): 728- 735. e 4. DOI: 10.1016/j.cgh.2019.11.012. [18] NAN Y, GARAY OU, LU X, et al. Early-stage hepatocellular carcinoma screening in patients with chronic hepatitis B in China: a cost-effectiveness analysis[J]. J Comp Eff Res, 2024, 13( 4): e230146. DOI: 10.57264/cer-2023-0146. [19] LOGLIO A, IAVARONE M, FACCHETTI F, et al. The combination of PIVKA-II and AFP improves the detection accuracy for HCC in HBV Caucasian cirrhotics on long-term oral therapy[J]. Liver Int, 2020, 40( 8): 1987- 1996. DOI: 10.1111/liv.14475. [20] KIM HS, PARK JW, JANG JS, et al. Prognostic values of alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II in hepatitis B virus-related hepatocellular carcinoma: A prospective study[J]. J Clin Gastroenterol, 2009, 43( 5): 482- 488. DOI: 10.1097/MCG.0b013e318182015a. [21] FENG HL, LI BL, LI Z, et al. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma[J]. BMC Cancer, 2021, 21( 1): 401. DOI: 10.1186/s12885-021-08138-3. -



 PDF下载 ( 917 KB)
PDF下载 ( 917 KB)

 下载:
下载: