地衣芽孢杆菌制剂对非酒精性脂肪性肝病大鼠模型肝脂肪变性及肠黏膜屏障功能的影响
DOI: 10.12449/JCH241012
Effect of Bacillus licheniformis preparation on hepatic steatosis and intestinal mucosal barrier function in a rat model of nonalcoholic fatty liver disease
-
摘要:
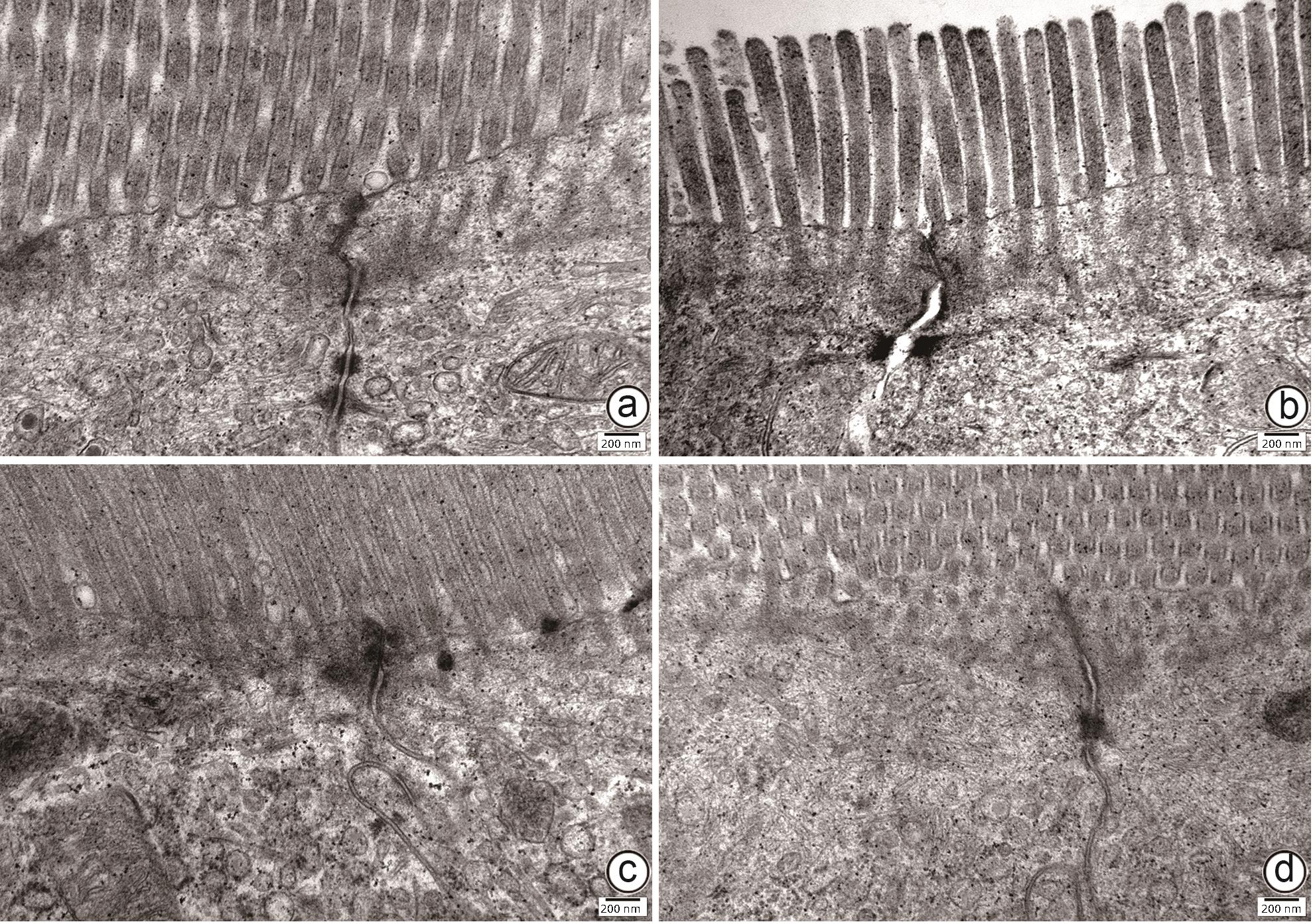
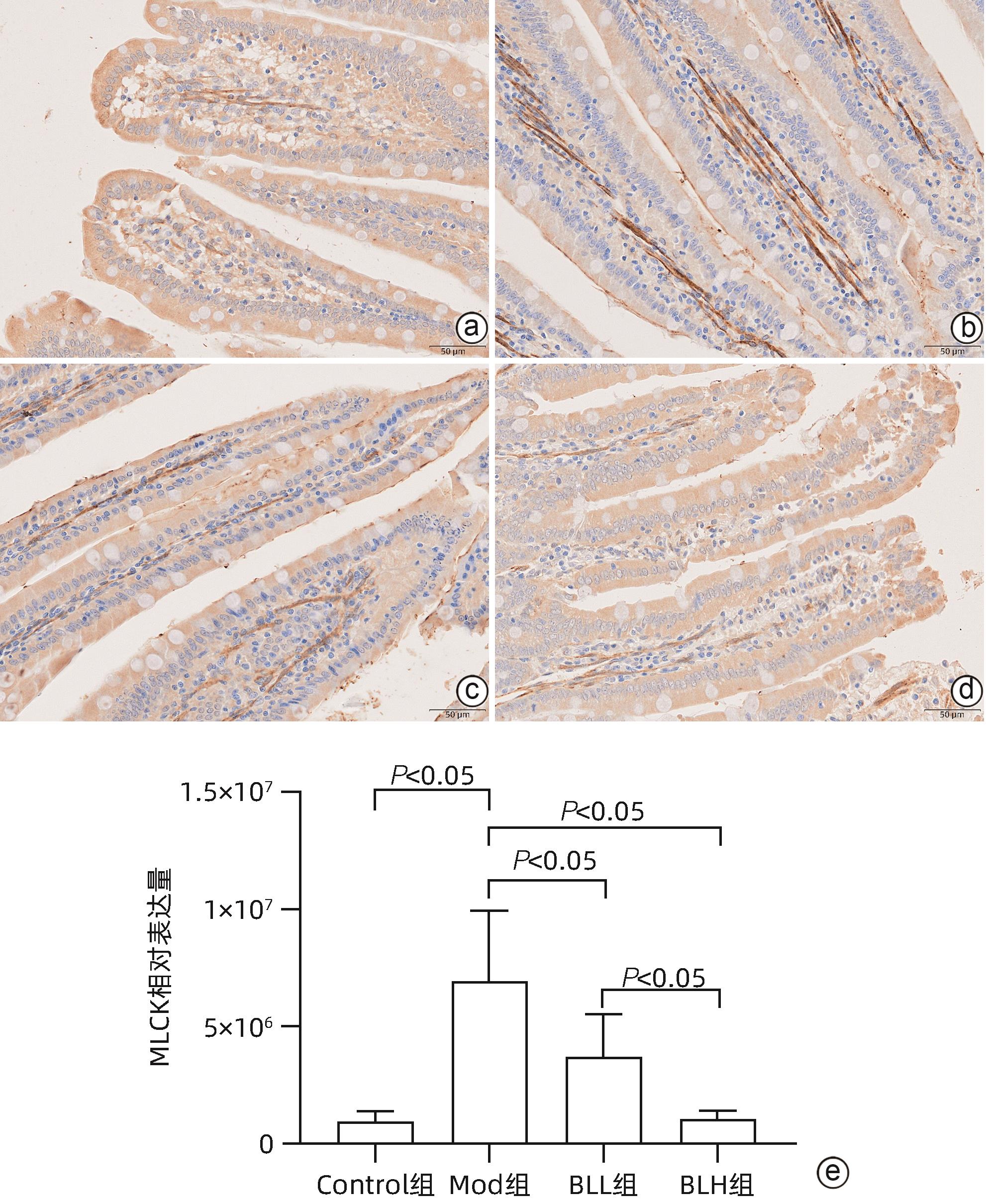
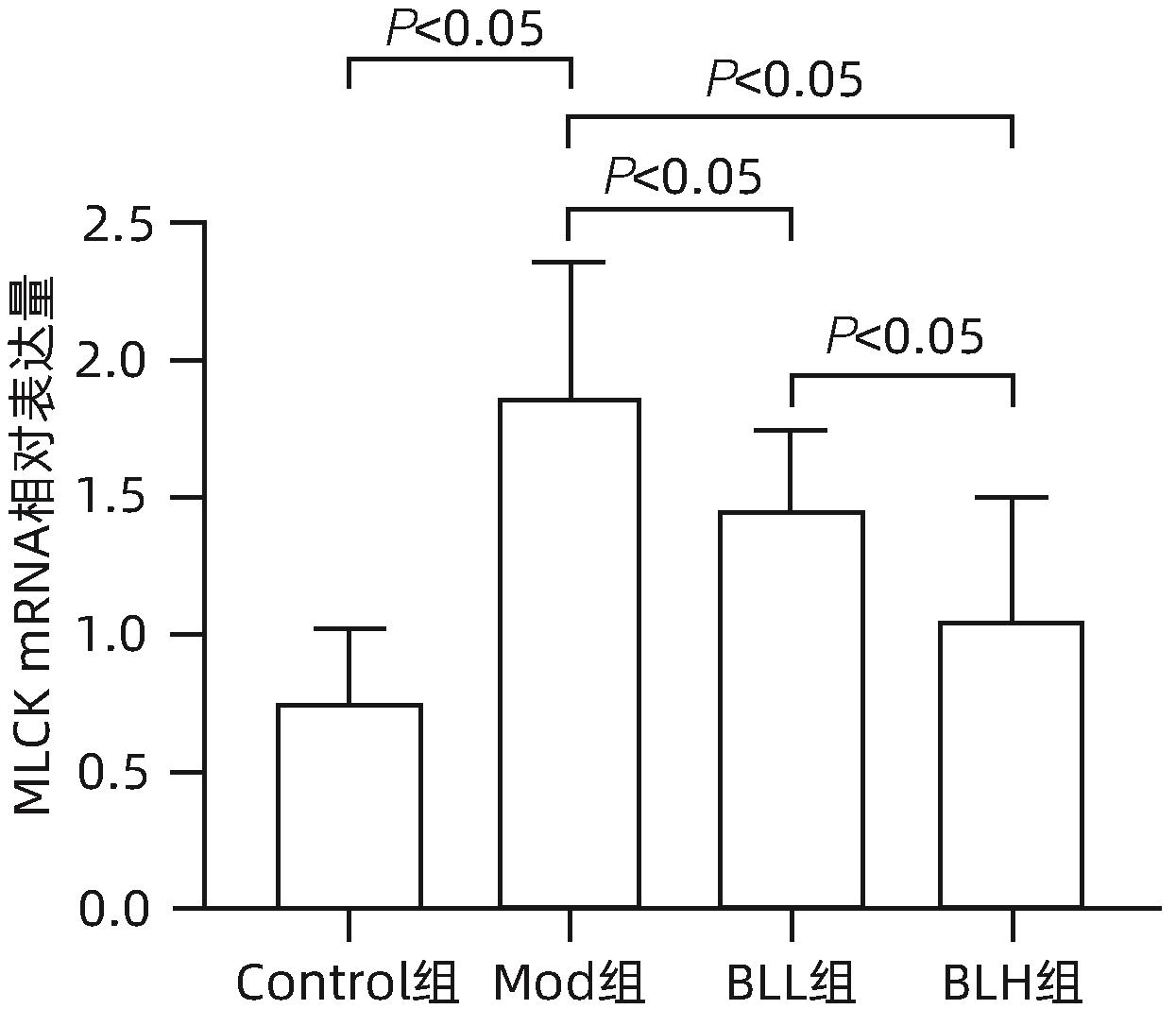
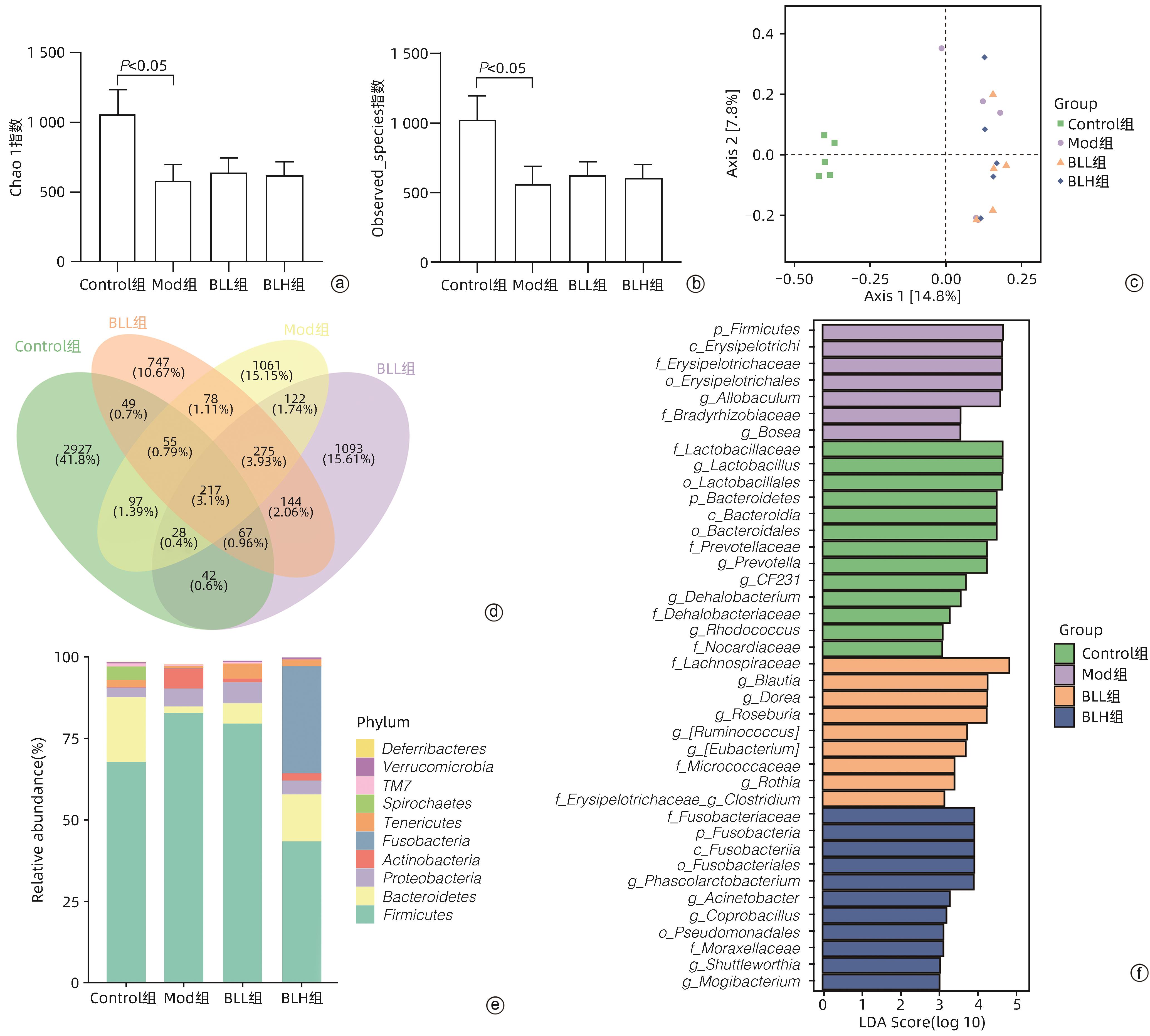
目的 通过建立非酒精性脂肪性肝病(NAFLD)大鼠模型,探讨地衣芽孢杆菌制剂对大鼠肝组织病理学及肠黏膜屏障功能的影响。 方法 将30只雄性SD大鼠随机分为正常对照组(Control组,n=5)、模型组(Mod组,n=15)、地衣芽孢杆菌低剂量组(BLL组,n=5)和地衣芽孢杆菌高剂量组(BLH组,n=5)。Control组给予普通饲料喂养,Mod组、BLL组、BLH组给予高脂饲料喂养16周,其中BLL组、BLH组在高脂饲料喂养8周后,分别予地衣芽孢杆菌制剂灌胃2.5×107 CFU/kg、5.0×107 CFU/kg,每天1次,持续8周。8周干预结束后,检测大鼠血清ALT、AST、TC、TG、空腹血糖(FPG)、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、丙二醛(MDA)、IL-6、IL-1β和TNF-α水平。HE染色观察肝组织病理学改变并根据NAFLD活动度积分(NAS)评分。透射电镜下观察肠黏膜紧密连接结构。免疫组化染色观察肠黏膜肌球蛋白轻链激酶(MLCK)的表达。实时定量PCR检测肠黏膜MLCK的mRNA水平。高通量16S rRNA测序法分析肠道菌群组成。采用单因素方差分析进行多组间比较,多重比较采用Bonferroni法。 结果 与Mod组相比,BLH组和BLL组大鼠血清ALT、AST、TC、TG、FPG、IL-1β及TNF-α水平降低,SOD水平升高,肝细胞脂肪变减轻,NAS评分降低,肠黏膜紧密连接结构恢复,MLCK蛋白及mRNA表达水平降低,差异均有统计学意义(P值均<0.05)。此外,BLH组较Mod组CAT水平升高,MDA及IL-6水平下降,差异均有统计学意义(P值均<0.05)。与BLL组相比,BLH组大鼠血清MDA、IL-6、TNF-α水平降低,CAT水平升高,肠黏膜MLCK蛋白及mRNA表达水平降低,差异均有统计学意义(P值均<0.05)。肠道菌群示BLH组和BLL组厚壁菌门和拟杆菌门的相对丰度均较Mod组有所恢复,且BLH组厚壁菌门和拟杆菌门的相对丰度更接近Control组。 结论 地衣芽孢杆菌制剂可有效减轻NAFLD大鼠的肝脂肪变性,其作用机制可能与下调肠黏膜MLCK蛋白的表达水平、改善肠黏膜紧密连接结构有关。 Abstract:Objective To investigate the effects of Bacillus licheniformis on liver histopathology and intestinal mucosal barrier function in rats with nonalcoholic fatty liver disease(NAFLD). Methods A total of 30 male Sprague-Dawley rats were randomly divided into normal control group (Control group with 5 rats), model group (Mod group with 15 rats), low-dose Bacillus licheniformis group (BLL group with 5 rats), and high-dose Bacillus licheniformis group (BLH group with 5 rats). The rats in the Control group were fed with normal diet, and those in the Mod, BLL, and BLH groups were fed with high-fat diet for 16 weeks; after 8 weeks of feeding with high-fat diet, the rats in the BLL and BLH groups were given Bacillus licheniformis preparation by gavage once a day for 8 consecutive weeks at a dose of 2.5×107 CFU/kg and 5.0×107 CFU/kg, respectively. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglyceride (TG), fasting plasma glucose (FPG), superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) were measured after the 8 weeks of intervention; HE staining was used to observe the histopathological changes of rat liver, and NAFLD activity score (NAS) was used for scoring; transmission electron microscopy was used to observe the tight junction of the intestinal mucosa; immunohistochemical staining was used to measure the expression of myosin light chain kinase (MLCK) in intestinal mucosa, and quantitative real-time PCR was used to measure the mRNA expressional level of MLCK in intestinal mucosa; high-throughput 16S rRNA sequencing was used to analyze the composition of intestinal microbiota. A one-way analysis of variance was used for comparison between groups, and the Bonferroni method was used for multiple comparisons. Results Compared with the Mod group, the BLH and BLL groups had significant reductions in the serum levels of ALT, AST, TC, TG, FPG, IL-1β, and TNF-α, a significant increase in the level of SOD, significant alleviation of hepatocyte steatosis, a significant reduction in NAS score, recovery of the tight junction of intestinal mucosa, and significant reductions in the protein and mRNA expression levels of MLCK (all P<0.05). In addition, compared with the Mod group, the BLH group had a significant increase in CAT and significant reductions in MDA and IL-6 (all P<0.05). Compared with the BLL group, the BLH group had significant reductions in the serum levels of MDA, IL-6, and TNF-α and a significant increase in CAT, as well as significant reductions in the protein and mRNA expression levels of MLCK (all P<0.05). The analysis of intestinal microbiota showed that compared with the Mod group, the BLH and BLL groups had recovery of the relative abundances of Firmicutes and Bacteroidetes, and the relative abundances of Firmicutes and Bacteroidetes in the BLH group were closer to those in the Control group. Conclusion Bacillus licheniformis preparation can effectively alleviate hepatic steatosis in NAFLD rats, possibly by downregulating the expression level of MLCK and improving the tight junction structure of intestinal mucosa. -
表 1 实时定量PCR引物
Table 1. Real-time quantitative PCR primers
引物名称 引物序列 扩增产物 MLCK L:5'-GGAGCCTGCTTAGTCACCGT-3' 146 bp R:5'-AGCCGATGCAGACCTTGTTC-3' GAPDH L:5'-TGTTCTAGAGACAGCCGCAT-3' 83 bp R:5'-AAATCCGTTCACACCGACCT-3' 表 2 各组体质量、肝功能、血脂、血糖、抗氧化酶及炎症因子的比较
Table 2. Comparison of body weight, liver function, blood lipid, blood glucose, antioxidant enzymes and inflammatory factors in each group
项目 Control组(n=5) Mod组(n=5) BLL组(n=5) BLH组(n=5) F值 P值 体质量(g) 539.50±40.71 836.50±56.401) 713.00±35.042) 650.17±42.262) 46.989 <0.001 ALT(U/L) 5.52±1.57 14.71±7.281) 7.23±1.802) 5.19±1.652) 10.675 <0.001 AST(U/L) 15.80±6.32 51.42±18.861) 30.77±8.932) 26.87±5.052) 22.162 <0.001 TG(mmol/L) 0.64±0.22 1.30±0.261) 0.68±0.242) 0.76±0.472) 20.913 <0.001 TC(mmol/L) 1.56±0.28 3.13±0.541) 2.09±0.302) 1.98±0.662) 33.634 <0.001 FPG(µmol/mL) 4.16±1.30 15.84±8.641) 8.49±7.202) 7.12±4.192) 11.612 <0.001 CAT(U/mL) 7.22±2.38 2.85±0.481) 3.91±0.45 7.27±2.802)3) 33.038 <0.001 SOD(U/mL) 17.15±1.15 9.41±4.371) 16.60±0.562) 17.84±1.692) 16.277 <0.001 MDA(nmol/mL) 9.09±0.96 17.37±6.871) 14.56±2.79 8.12±1.712)3) 25.869 <0.001 TNF-α(pg/mL) 51.97±5.96 86.34±8.831) 64.00±6.642) 54.18±4.292)3) 56.092 <0.001 IL-6(pg/mL) 118.89±6.88 161.46±27.251) 143.85±19.29 120.70±9.272)3) 11.123 <0.001 IL-1β(pg/mL) 60.32±5.81 83.89±9.271) 67.76±2.472) 58.40±16.372) 15.381 <0.001 注:1)与Control组比较,P<0.05;2)与Mod组比较,P<0.05;3)与BLL组比较,P<0.05。
-
[1] ZHANG XY, LIU Y, WANG WL, et al. Diagnosis and evaluation of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 8): 1780- 1788. DOI: 10.3969/j.issn.1001-5256.2023.08.003.张馨元, 刘宇, 王文玲, 等. 非酒精性脂肪性肝病的诊断与评估[J]. 临床肝胆病杂志, 2023, 39( 8): 1780- 1788. DOI: 10.3969/j.issn.1001-5256.2023.08.003. [2] FANG J, YU CH, LI XJ, et al. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications[J]. Front Cell Infect Microbiol, 2022, 12: 997018. DOI: 10.3389/fcimb.2022.997018. [3] TILG H, ADOLPH TE, DUDEK M, et al. Non-alcoholic fatty liver disease: The interplay between metabolism, microbes and immunity[J]. Nat Metab, 2021, 3( 12): 1596- 1607. DOI: 10.1038/s42255-021-00501-9. [4] WU JY, WANG K, WANG XM, et al. The role of the gut microbiome and its metabolites in metabolic diseases[J]. Protein Cell, 2021, 12( 5): 360- 373. DOI: 10.1007/s13238-020-00814-7. [5] MOHAMAD NOR MH, AYOB N, MOKHTAR NM, et al. The effect of probiotics(MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease[J]. Nutrients, 2021, 13( 9): 3192. DOI: 10.3390/nu13093192. [6] HE WQ, WANG J, SHENG JY, et al. Contributions of myosin light chain kinase to regulation of epithelial paracellular permeability and mucosal homeostasis[J]. Int J Mol Sci, 2020, 21( 3): 993. DOI: 10.3390/ijms21030993. [7] FILIPOVIC B, LUKIC S, MIJAC D, et al. The new therapeutic approaches in the treatment of non-alcoholic fatty liver disease[J]. Int J Mol Sci, 2021, 22( 24): 13219. DOI: 10.3390/ijms222413219. [8] HONG Y, SHENG LL, ZHONG J, et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice[J]. Gut Microbes, 2021, 13( 1): 1- 20. DOI: 10.1080/19490976.2021.1930874. [9] WEI M, XIA L, PING J, et al. Effects of Bacillus Licheniformis capsule on non-alcoholic fatty liver disease[J]. Chin J Integr Tradit West Med Dig, 2018, 26( 1): 54- 57. DOI: 10.3969/j.issn.1671-038X.2018.01.10.魏敏, 夏露, 平晶, 等. 地衣芽孢杆菌活菌胶囊治疗非酒精性脂肪性肝病的疗效评价[J]. 中国中西医结合消化杂志, 2018, 26( 1): 54- 57. DOI: 10.3969/j.issn.1671-038X.2018.01.10. [10] TINCOPA MA, LOOMBA R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis[J]. Lancet Gastroenterol Hepatol, 2023, 8( 7): 660- 670. DOI: 10.1016/S2468-1253(23)00066-3. [11] LIANG YJ, LIANG S, ZHANG YP, et al. Oral administration of compound probiotics ameliorates HFD-induced gut microbe dysbiosis and chronic metabolic inflammation via the G protein-coupled receptor 43 in non-alcoholic fatty liver disease rats[J]. Probiotics Antimicrob Proteins, 2019, 11( 1): 175- 185. DOI: 10.1007/s12602-017-9378-3. [12] WANG W, XU AL, LI ZC, et al. Combination of probiotics and Salvia miltiorrhiza polysaccharide alleviates hepatic steatosis via gut microbiota modulation and insulin resistance improvement in high fat-induced NAFLD mice[J]. Diabetes Metab J, 2020, 44( 2): 336- 348. DOI: 10.4093/dmj.2019.0042. [13] SINGH RP, SHADAN A, MA Y. Biotechnological applications of probiotics: A multifarious weapon to disease and metabolic abnormality[J]. Probiotics Antimicrob Proteins, 2022, 14( 6): 1184- 1210. DOI: 10.1007/s12602-022-09992-8. [14] KAUFMANN B, SEYFRIED N, HARTMANN D, et al. Probiotics, prebiotics, and synbiotics in nonalcoholic fatty liver disease and alcohol-associated liver disease[J]. Am J Physiol Gastrointest Liver Physiol, 2023, 325( 1): G42- G61. DOI: 10.1152/ajpgi.00017.2023. [15] LI HT, WANG W, ZHANG ZJ. The effect of Bnfillus licheniformis on nonalcoholic fatty liver disease[J]. China Med Her, 2012, 9( 3): 154- 155. DOI: 10.3969/j.issn.1673-7210.2012.03.070.李海涛, 王雯, 张志坚. 地衣芽孢杆菌对非酒精性脂肪性肝病的治疗作用[J]. 中国医药导报, 2012, 9( 3): 154- 155. DOI: 10.3969/j.issn.1673-7210.2012.03.070. [16] SONG XM, WU XD, SHI K, et al. Effect of Bacillus licheniformis on non-alcoholic fatty liver disease and intestinal permeability in rats[J]. J Pract Med, 2018, 34( 24): 4056- 4059. DOI: 10.3969/j.issn.1006-5725.2018.24.010.宋献美, 吴晓东, 石科, 等. 地衣芽孢杆菌对非酒精性脂肪肝病的干预作用及对肠黏膜通透性的影响[J]. 实用医学杂志, 2018, 34( 24): 4056- 4059. DOI: 10.3969/j.issn.1006-5725.2018.24.010. [17] DUAN YM, PAN XF, LUO JY, et al. Association of inflammatory cytokines with non-alcoholic fatty liver disease[J]. Front Immunol, 2022, 13: 880298. DOI: 10.3389/fimmu.2022.880298. [18] CHEN JH, ZHOU H, JIN HW, et al. Role of inflammatory factors in mediating the effect of lipids on nonalcoholic fatty liver disease: A two-step, multivariable Mendelian randomization study[J]. Nutrients, 2022, 14( 20): 4434. DOI: 10.3390/nu14204434. [19] VACHLIOTIS ID, POLYZOS SA. The role of tumor necrosis factor-alpha in the pathogenesis and treatment of nonalcoholic fatty liver disease[J]. Curr Obes Rep, 2023, 12( 3): 191- 206. DOI: 10.1007/s13679-023-00519-y. [20] KARKUCINSKA-WIECKOWSKA A, SIMOES ICM, KALINOWSKI P, et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship[J]. Eur J Clin Invest, 2022, 52( 3): e13622. DOI: 10.1111/eci.13622. [21] ALLAMEH A, NIAYESH-MEHR R, ALIARAB A, et al. Oxidative stress in liver pathophysiology and disease[J]. Antioxidants, 2023, 12( 9): 1653. DOI: 10.3390/antiox12091653. [22] CAMILLERI M. Leaky gut: Mechanisms, measurement and clinical implications in humans[J]. Gut, 2019, 68( 8): 1516- 1526. DOI: 10.1136/gutjnl-2019-318427. [23] JIN Y, BLIKSLAGER AT. The regulation of intestinal mucosal barrier by myosin light chain kinase/rho kinases[J]. Int J Mol Sci, 2020, 21( 10): 3550. DOI: 10.3390/ijms21103550. [24] LIU L, YIN MY, GAO JW, et al. Intestinal barrier function in the pathogenesis of nonalcoholic fatty liver disease[J]. J Clin Transl Hepatol, 2023, 11( 2): 452- 458. DOI: 10.14218/JCTH.2022.00089. [25] HU HM, LIN AZ, KONG MW, et al. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives[J]. J Gastroenterol, 2020, 55( 2): 142- 158. DOI: 10.1007/s00535-019-01649-8. [26] SUN JY, FAN JM, LI TT, et al. Nuciferine protects against high-fat diet-induced hepatic steatosis via modulation of gut microbiota and bile acid metabolism in rats[J]. J Agric Food Chem, 2022, 70( 38): 12014- 12028. DOI: 10.1021/acs.jafc.2c04817. [27] LI XL, SUN FX, ZHANG YX, et al. Analysis of intestinal flora in rats with non-alcoholic fatty liver disease based on 16S rRNA gene sequencing[J/CD]. Chin J Liver Dis Electron Version, 2023, 15( 4): 39- 46.李晓玲, 孙凤霞, 张莹雪, 等. 基于16S rRNA基因测序技术分析非酒精性脂肪性肝病大鼠的肠道菌群[J/CD]. 中国肝脏病杂志(电子版), 2023, 15( 4): 39- 46. [28] SONG W, WANG TY, CUI XL, et al. Lactobacillus coryniformis subsp. torquens T3 alleviates non-alcoholic fatty liver disease via reconstruction of the gut microbiota and redox system[J]. J Sci Food Agric, 2023, 103( 14): 6814- 6825. DOI: 10.1002/jsfa.12774. [29] PAN XF, WEN SW, KAMINGA AC, et al. Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Sci Rep, 2020, 10( 1): 8848. DOI: 10.1038/s41598-020-65051-8. -



 PDF下载 ( 4213 KB)
PDF下载 ( 4213 KB)


 下载:
下载: