基于双向孟德尔随机化的不同BMI分型非酒精性脂肪性肝病与2型糖尿病的遗传关联分析
DOI: 10.12449/JCH241011
The genetic association between nonalcoholic fatty liver disease and type 2 diabetes mellitus in different body mass index categories: A bidirectional Mendelian randomization study
-
摘要:
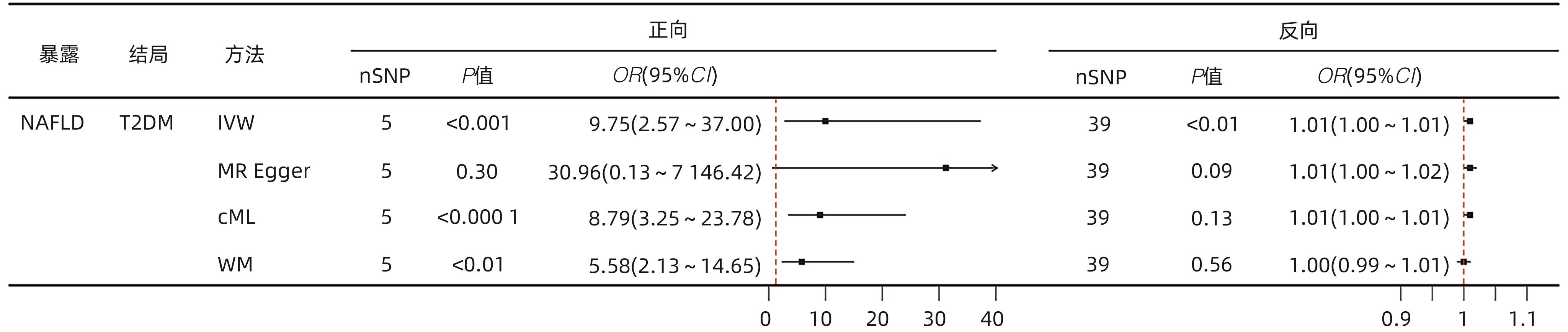
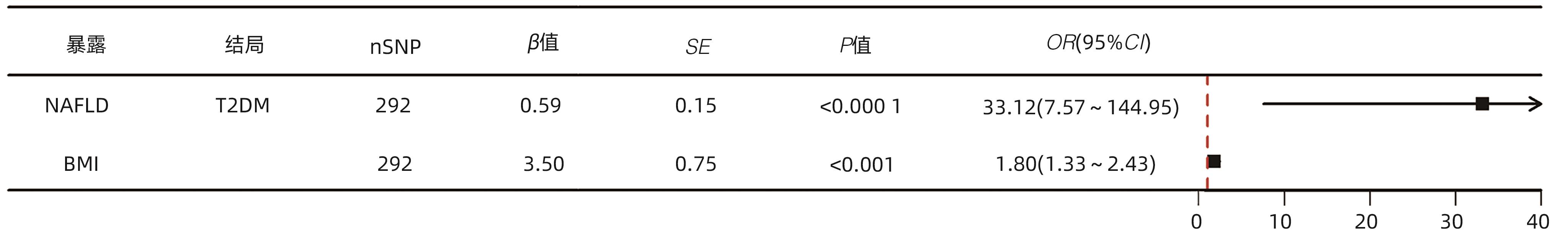
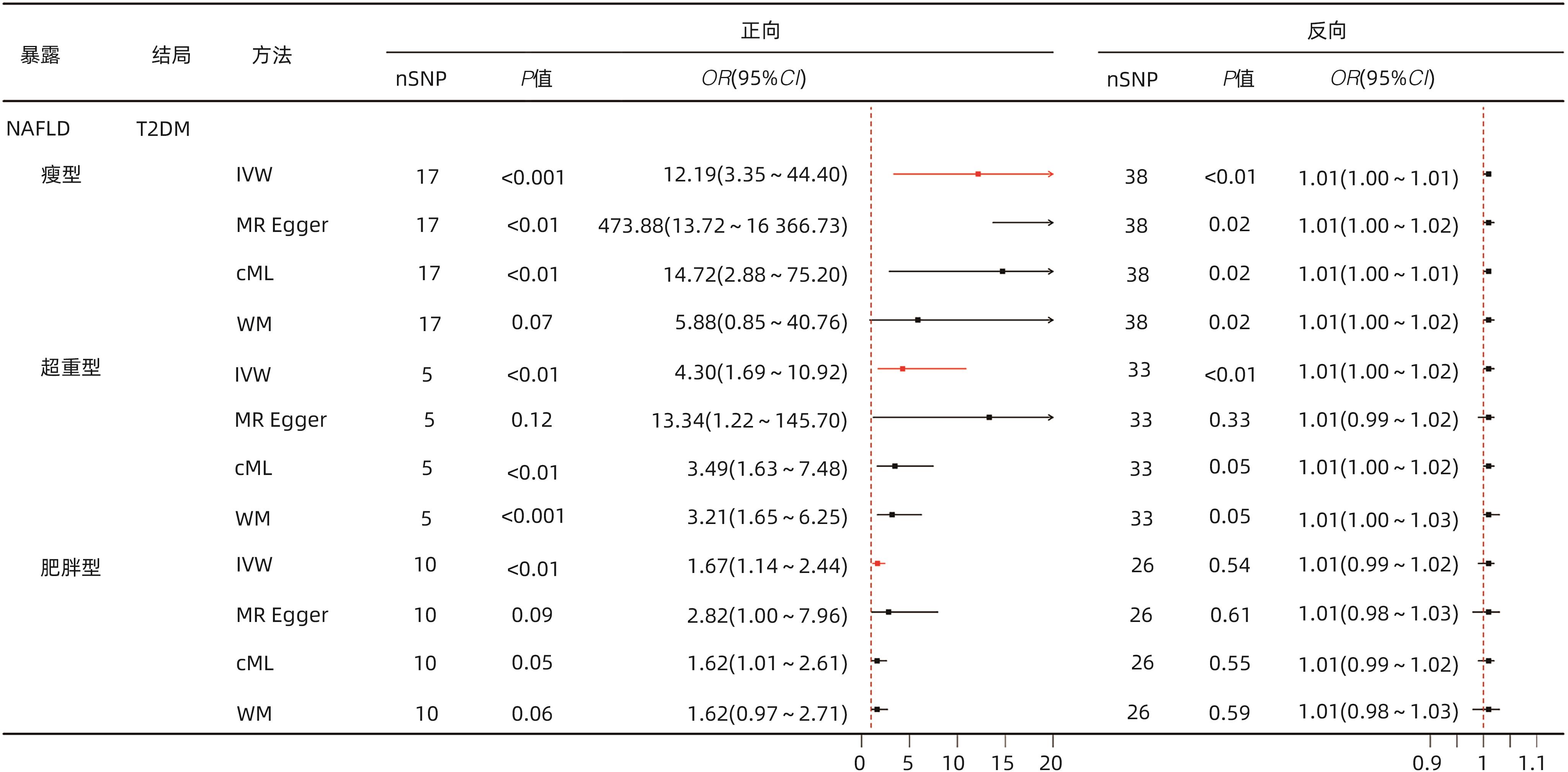
目的 运用双向双样本孟德尔随机化(MR)评估非酒精性脂肪性肝病(NAFLD)与2型糖尿病(T2DM)的遗传关联,并进一步探讨不同BMI的NAFLD人群与T2DM的因果关系。 方法 数据来源于以欧洲人群为研究对象的全基因组关联研究,其中NAFLD的样本量为32 941例,T2DM为312 646例,BMI为681 275例。运用单变量、多变量MR方法评估NAFLD总人群及各BMI亚型与T2DM之间的双向因果关系。采用逆方差加权法、MR-Egger回归、约束最大似然与模型平均法、加权中位数法进行MR分析,采用MR多效性残差和与离群值、径向MR、MR-Egger截距法、Cochran Q检验进行敏感性分析。 结果 单变量MR分析显示NAFLD总人群与T2DM之间存在双向因果关系(正向OR=9.75,95%CI:2.57~37.00,P<0.001;反向OR=1.01,95%CI:1.00~1.01,P<0.01)。多变量MR分析显示经BMI校正后,NAFLD总人群与T2DM的因果关系仍然保持显著(OR=33.12,95%CI:7.57~144.95,P<0.000 1)。亚组分析显示,NAFLD各亚组均与T2DM存在因果关系(瘦型OR=12.19,95%CI:3.35~44.40,P<0.001;超重型OR=4.30,95%CI:1.69~10.92,P<0.01;肥胖型OR=1.67,95%CI:1.14~2.44,P<0.01)。 结论 本研究从遗传学层面揭示了NAFLD总人群及各BMI亚型与T2DM之间的因果关系。 Abstract:Objective To investigate the genetic association between nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) using bidirectional two-sample Mendelian randomization (MR), as well as the causal relationship between NAFLD and T2DM across different body mass index (BMI) categories. Methods The data were derived from genome-wide association studies conducted in European populations, with a sample size of 32 941 cases for NAFLD, 312 646 cases for T2DM, and 681 275 cases for BMI. The univariate and multivariate MR methods were used to assess the bidirectional causal relationship between NAFLD and T2DM in the general population and across different BMI subtypes. The methods of inverse-variance weighting, MR-Egger regression, constrained maximum likelihood and model averaging, and weighted median were used to conduct the MR analysis, and MR-Pleiotropy Residual Sum and Outlier, radial MR, the MR-Egger intercept method, and the Cochrane Q test were used for sensitivity analysis. Results The univariate MR analysis revealed a bidirectional causal relationship between NAFLD and T2DM in the general population (forward analysis: odds ratio [OR]=9.75, 95% confidence interval [CI]: 2.57 — 37.00, P<0.001; reverse analysis: OR=1.01, 95%CI: 1.00 — 1.01, P<0.01). After adjustment for BMI, the multivariate MR analysis showed that the causal relationship between NAFLD and T2DM remained significant in the general population (OR=33.12, 95%CI: 7.57 — 144.95, P<0.000 1). The subgroup analysis showed a causal relationship between NAFLD and T2DM across all BMI subtypes (lean subgroup: OR=12.19, 95%CI: 3.35 — 44.40, P<0.001; overweight subgroup: OR=4.30, 95%CI: 1.69 — 10.92, P<0.01; obese subgroup: OR=1.67, 95%CI: 1.14 — 2.44, P<0.01). Conclusion This study reveals the causal relationship between NAFLD and T2DM in the general population of NAFLD and across different BMI subtypes from a genetic perspective. -
表 1 各表型GWAS数据汇总信息
Table 1. Summary information of GWAS data for each phenotype
表型 数据来源 PMID 样本量(例) 种族 NAFLD(总人群) UKB 37235137 32 941 欧洲 瘦型NAFLD UKB 37235137 13 614 欧洲 超重型NAFLD UKB 37235137 13 710 欧洲 肥胖型NAFLD UKB 37235137 5 617 欧洲 T2DM CMDKP 34862199 312 646 欧洲 BMI GIANT 30124842 681 275 欧洲 注:PMID,PubMed唯一标识码。
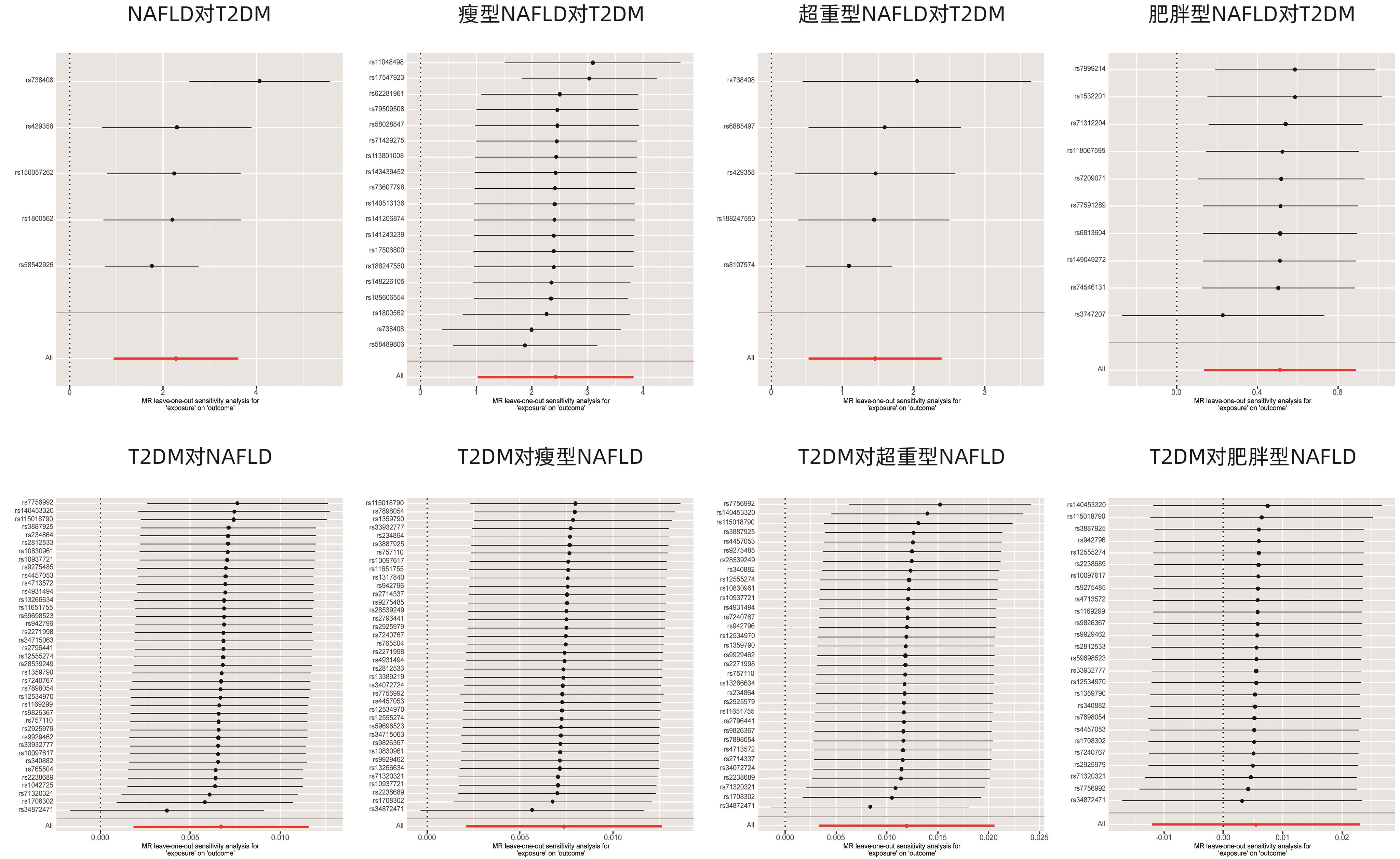
表 2 NAFLD各组与T2DM的敏感性分析
Table 2. Sensitivity analysis of NAFLD subgroups and T2DM
暴露 结局 正向 反向 异质性(P值) 水平多效性 异质性(P值) 水平多效性 MR-Egger截距法P值 MR-PRESSO P值 MR-PRESSO 剔除SNP MR-Egger截距法P值 MR-PRESSO P值 MR-PRESSO 剔除SNP NAFLD总人群 T2DM 0.02 0.69 0.32 无 0.41 0.91 0.38 无 瘦型NAFLD T2DM 0.98 0.05 0.71 rs17547923,
rs584898061)
0.99 0.30 0.99 无 超重型NAFLD T2DM 0.04 0.39 0.21 无 0.61 0.40 0.58 无 肥胖型NAFLD T2DM 0.74 0.32 0.60 无 1.00 0.87 1.00 无 注:1)第一次MR-Egger截距法P=0.002,进一步采用径向MR剔除的SNP离群值。
-
[1] POWELL EE, WONG VWS, RINELLA M. Non-alcoholic fatty liver disease[J]. Lancet, 2021, 397( 10290): 2212- 2224. DOI: 10.1016/S0140-6736(20)32511-3. [2] RIAZI K, AZHARI H, CHARETTE JH, et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2022, 7( 9): 851- 861. DOI: 10.1016/S2468-1253(22)00165-0. [3] LI L, LIU DW, YAN HY, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: Evidence from a meta-analysis of 21 cohort studies[J]. Obes Rev, 2016, 17( 6): 510- 519. DOI: 10.1111/obr.12407. [4] NABI O, LAPIDUS N, BOURSIER J, et al. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes(NASH-CO Study)[J]. Hepatology, 2023, 78( 1): 272- 283. DOI: 10.1097/HEP.0000000000000329. [5] XU R, PAN J, ZHOU W, et al. Recent advances in lean NAFLD[J]. Biomed Pharmacother, 2022, 153: 113331. DOI: 10.1016/j.biopha.2022.113331. [6] TANG A, NG CH, PHANG PH, et al. Comparative burden of metabolic dysfunction in lean NAFLD vs non-lean NAFLD-A systematic review and meta-analysis[J]. Clin Gastroenterol Hepatol, 2023, 21( 7): 1750- 1760. e 12. DOI: 10.1016/j.cgh.2022.06.029. [7] International Diabetes Federation. IDF Diabetes Atlas(10th ed)[M]. Brussels: International Diabetes Federation, 2022. [8] YOUNOSSI ZM, GOLABI P, PRICE JK, et al. The global epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among patients with type 2 diabetes[J]. Clin Gastroenterol Hepatol, 2024. DOI: 10.1016/j.cgh.2024.03.006.[ Online ahead of print] [9] CAO LM, AN Y, LIU HY, et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: A systematic review and meta-analysis[J]. BMC Med, 2024, 22( 1): 101. DOI: 10.1186/s12916-024-03315-0. [10] GAO YT, ZHAO TY, SONG SN, et al. Lean nonalcoholic fatty liver disease and risk of incident type 2 diabetes mellitus: A literature review and meta-analysis[J]. Diabetes Res Clin Pract, 2023, 200: 110699. DOI: 10.1016/j.diabres.2023.110699. [11] BYRNE CD, TARGHER G. NAFLD: A multisystem disease[J]. J Hepatol, 2015, 62( 1 suppl): S47- S64. DOI: 10.1016/j.jhep.2014.12.012. [12] SEKULA P, FABIOLA GRECO M, PATTARO C, et al. Mendelian randomization as an approach to assess causality using observational data[J]. J Am Soc Nephrol, 2016, 27( 11): 3253- 3265. DOI: 10.1681/ASN.2016010098. [13] FERENCE BA, HOLMES MV, SMITH GD. Using Mendelian randomization to improve the design of randomized trials[J]. Cold Spring Harb Perspect Med, 2021, 11( 7): a040980. DOI: 10.1101/cshperspect.a040980. [14] SKRIVANKOVA VW, RICHMOND RC, WOOLF BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: The STROBE-MR statement[J]. JAMA, 2021, 326( 16): 1614- 1621. DOI: 10.1001/jama.2021.18236. [15] SUN ZW, PAN XC, TIAN AW, et al. Genetic variants in HFE are associated with non-alcoholic fatty liver disease in lean individuals[J]. JHEP Rep, 2023, 5( 7): 100744. DOI: 10.1016/j.jhepr.2023.100744. [16] O’CONNOR MJ, SCHROEDER P, HUERTA-CHAGOYA A, et al. Recessive genome-wide meta-analysis illuminates genetic architecture of type 2 diabetes[J]. Diabetes, 2022, 71( 3): 554- 565. DOI: 10.2337/db21-0545. [17] YENGO L, SIDORENKO J, KEMPER KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry[J]. Hum Mol Genet, 2018, 27( 20): 3641- 3649. DOI: 10.1093/hmg/ddy271. [18] HEMANI G, TILLING K, DAVEY SMITH G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data[J]. PLoS Genet, 2017, 13( 11): e1007081. DOI: 10.1371/journal.pgen.1007081. [19] BURGESS S, SCOTT RA, TIMPSON NJ, et al. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors[J]. Eur J Epidemiol, 2015, 30( 7): 543- 552. DOI: 10.1007/s10654-015-0011-z. [20] BOWDEN J, DAVEY SMITH G, BURGESS S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression[J]. Int J Epidemiol, 2015, 44( 2): 512- 525. DOI: 10.1093/ije/dyv080. [21] XUE HR, SHEN XT, PAN W. Constrained maximum likelihood-based Mendelian randomization robust to both correlated and uncorrelated pleiotropic effects[J]. Am J Hum Genet, 2021, 108( 7): 1251- 1269. DOI: 10.1016/j.ajhg.2021.05.014. [22] BOWDEN J, DAVEY SMITH G, HAYCOCK PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted Median estimator[J]. Genet Epidemiol, 2016, 40( 4): 304- 314. DOI: 10.1002/gepi.21965. [23] SANDERSON E, DAVEY SMITH G, WINDMEIJER F, et al. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings[J]. Int J Epidemiol, 2019, 48( 3): 713- 727. DOI: 10.1093/ije/dyy262. [24] BURGESS S, THOMPSON SG. Interpreting findings from Mendelian randomization using the MR-Egger method[J]. Eur J Epidemiol, 2017, 32( 5): 377- 389. DOI: 10.1007/s10654-017-0255-x. [25] VERBANCK M, CHEN CY, NEALE B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases[J]. Nat Genet, 2018, 50( 5): 693- 698. DOI: 10.1038/s41588-018-0099-7. [26] BOWDEN J, SPILLER W, DEL GRECO M F, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression[J]. Int J Epidemiol, 2018, 47( 6): 2100. DOI: 10.1093/ije/dyy265. [27] BOWDEN J, DEL GRECO M F, MINELLI C, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: Moving beyond the NOME assumption[J]. Int J Epidemiol, 2019, 48( 3): 728- 742. DOI: 10.1093/ije/dyy258. [28] BURGESS S, BOWDEN J, FALL T, et al. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants[J]. Epidemiology, 2017, 28( 1): 30- 42. DOI: 10.1097/EDE.0000000000000559. [29] Fatty Liver and Alcoholic Liver Disease Group, Hepatology Branch of Chinese Medical Association. Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases[J]. J Clin Hepatol, 2010, 2( 4): 43- 48. DOI: 10.3969/j.issn.1674-7380.2010.04.013.中华医学会肝脏病学分会脂肪肝和酒精性肝病学组. 非酒精性脂肪性肝病诊疗指南[J]. 临床肝胆病杂志, 2010, 26( 2): 120- 124. DOI: 10.3760/cma.j.jssn.1674-5809.2010.01.000. [30] LIU ZP, ZHANG Y, GRAHAM S, et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping[J]. J Hepatol, 2020, 73( 2): 263- 276. DOI: 10.1016/j.jhep.2020.03.006. [31] NI XT, TONG C, HALENGBIEKE A, et al. Association between nonalcoholic fatty liver disease and type 2 diabetes: A bidirectional two-sample Mendelian randomization study[J]. Diabetes Res Clin Pract, 2023, 206: 110993. DOI: 10.1016/j.diabres.2023.110993. [32] MANTOVANI A, BYRNE CD, BONORA E, et al. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: A meta-analysis[J]. Diabetes Care, 2018, 41( 2): 372- 382. DOI: 10.2337/dc17-1902. [33] MANTOVANI A, PETRACCA G, BEATRICE G, et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals[J]. Gut, 2021, 70( 5): 962- 969. DOI: 10.1136/gutjnl-2020-322572. [34] KOSMALSKI M, ŚLIWIŃSKA A, DRZEWOSKI J. Non-alcoholic fatty liver disease or type 2 diabetes mellitus-the chicken or the egg dilemma[J]. Biomedicines, 2023, 11( 4): 1097. DOI: 10.3390/biomedicines11041097. [35] KHAN RS, BRIL F, CUSI K, et al. Modulation of insulin resistance in nonalcoholic fatty liver disease[J]. Hepatology, 2019, 70( 2): 711- 724. DOI: 10.1002/hep.30429. [36] ZHANG XY, LIU Y, WANG WL, et al. Diagnosis and evaluation of nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 8): 1780- 1788. DOI: 10.3969/j.issn.1001-5256.2023.08.003.张馨元, 刘宇, 王文玲, 等. 非酒精性脂肪性肝病的诊断与评估[J]. 临床肝胆病杂志, 2023, 39( 8): 1780- 1788. DOI: 10.3969/j.issn.1001-5256.2023.08.003. [37] ESLAM M, VALENTI L, ROMEO S. Genetics and epigenetics of NAFLD and NASH: Clinical impact[J]. J Hepatol, 2018, 68( 2): 268- 279. DOI: 10.1016/j.jhep.2017.09.003. [38] LIU DJ, PELOSO GM, YU HJ, et al. Exome-wide association study of plasma lipids in>300, 000 individuals[J]. Nat Genet, 2017, 49( 12): 1758- 1766. DOI: 10.1038/ng.3977. [39] BESSONE F, RAZORI MV, ROMA MG. Molecular pathways of nonalcoholic fatty liver disease development and progression[J]. Cell Mol Life Sci, 2019, 76( 1): 99- 128. DOI: 10.1007/s00018-018-2947-0. [40] MALONE JI, HANSEN BC. Does obesity cause type 2 diabetes mellitus(T2DM)? Or is it the opposite?[J]. Pediatr Diabetes, 2019, 20( 1): 5- 9. DOI: 10.1111/pedi.12787. [41] YE Q, ZOU BY, YEO YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis[J]. Lancet Gastroenterol Hepatol, 2020, 5( 8): 739- 752. DOI: 10.1016/S2468-1253(20)30077-7. [42] PINGITORE P, ROMEO S. The role of PNPLA3 in health and disease[J]. Biochim Biophys Acta Mol Cell Biol Lipids, 2019, 1864( 6): 900- 906. DOI: 10.1016/j.bbalip.2018.06.018. [43] ZHANG YJ, ZHOU XQ. Research advances in lean nonalcoholic fatty liver disease[J]. J Clin Hepatol, 2023, 39( 12): 2914- 2919. DOI: 10.3969/j.issn.1001-5256.2023.12.024.张瑜娟, 周希乔. 瘦型非酒精性脂肪性肝病的研究进展[J]. 临床肝胆病杂志, 2023, 39( 12): 2914- 2919. DOI: 10.3969/j.issn.1001-5256.2023.12.024. [44] SMITH GI, POLIDORI DC, YOSHINO M, et al. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics[J]. J Clin Invest, 2020, 130( 6): 3305- 3314. DOI: 10.1172/JCI136756. [45] TOBARI M, HASHIMOTO E, TANIAI M, et al. Characteristics of non-alcoholic steatohepatitis among lean patients in Japan: Not uncommon and not always benign[J]. J Gastroenterol Hepatol, 2019, 34( 8): 1404- 1410. DOI: 10.1111/jgh.14585. [46] PETTA S, CIMINNISI S, DI MARCO V, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease[J]. Aliment Pharmacol Ther, 2017, 45( 4): 510- 518. DOI: 10.1111/apt.13889. [47] ZHOU XM, YU XY, SONG ZY. Clinical characteristics and management of lean nonalcoholic fatty liver disease[J]. Chin J Health Manag, 2024, 18( 3): 236- 240. DOI: 10.3760/cma.j.cn115624-20230803-00047.周馨媚, 余馨妍, 宋震亚. 瘦型非酒精性脂肪性肝病的临床特点和管理[J]. 中华健康管理学杂志, 2024, 18( 3): 236- 240. DOI: 10.3760/cma.j.cn115624-20230803-00047. [48] LI N, XANG W, WU SL, et al. Association between the lean nonalcoholic fatty liver disease and risk of incident type 2 diabetes in a healthy population of Northwest China: A retrospective cohort study with a 2-year follow-up period[J]. Front Endocrinol, 2023, 14: 1173757. DOI: 10.3389/fendo.2023.1173757. [49] FRACANZANI AL, PETTA S, LOMBARDI R, et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity[J]. Clin Gastroenterol Hepatol, 2017, 15( 10): 1604- 1611. e 1. DOI: 10.1016/j.cgh.2017.04.045. -



 PDF下载 ( 3023 KB)
PDF下载 ( 3023 KB)


 下载:
下载: