代谢相关因素对HBV相关慢加急性肝衰竭患者短期预后的影响及预测模型构建
DOI: 10.12449/JCH241010
Influence of metabolism-related factors on the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure and establishment of a predictive model
-
摘要:
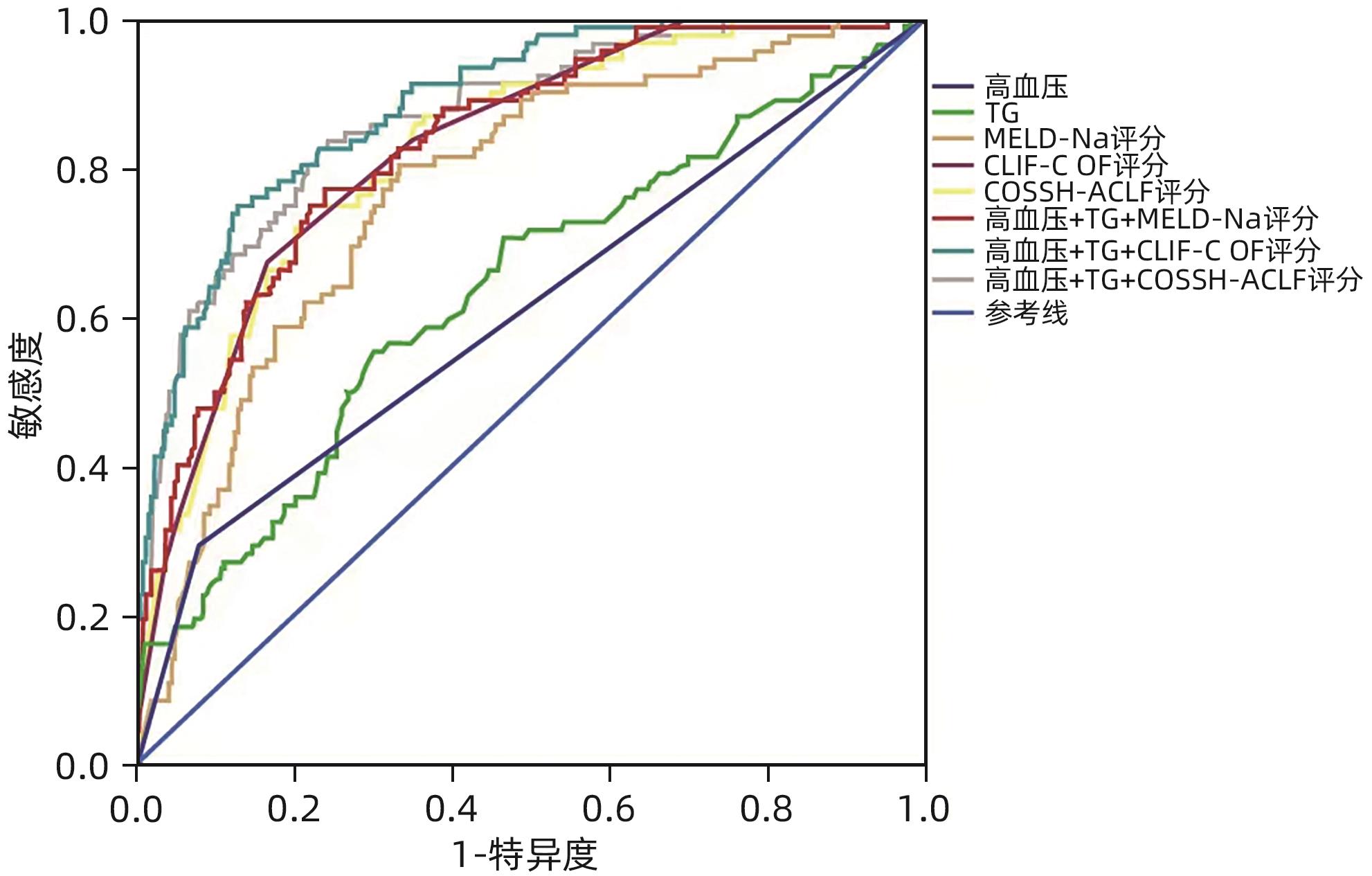
目的 本研究旨在评估代谢相关因素(超重和/或肥胖、高血糖、高血压及血脂紊乱)对HBV相关慢加急性肝衰竭(HBV-ACLF)患者90天预后的影响及预测模型构建。 方法 回顾性纳入2018年6月—2022年6月西南医科大学附属医院感染科收治的365例HBV-ACLF患者,根据90天随访结果分为生存组(n=273)和死亡组(n=92);收集患者的一般资料及相关实验室检查指标。符合正态分布的计量资料两组间比较采用成组t检验,非正态分布的计量资料两组间比较采用Mann-Whitney U检验。计数资料两组间比较采用χ2检验。采用Logistic回归分析代谢相关因素是否为HBV-ACLF患者90天预后的独立危险因素,Kaplan-Meier法分析检验代谢相关因素与HBV-ALCF患者90天生存率的关系。采用受试者工作特征曲线下面积(AUC)比较不同评分模型对HBV-ACLF患者90天预后的预测价值。 结果 多因素Logistic回归分析结果显示,高血压(OR=4.698,95%CI:1.904~11.593,P=0.001)、ALT(OR=0.999,95%CI:0.999~1.000,P=0.010)、TG(OR=4.979,95%CI:2.433~10.189,P<0.001)、HDL-C(OR=0.258,95%CI:0.087~0.762,P=0.012)、载脂蛋白B(OR=0.118,95%CI:0.026~0.547,P=0.006)、CLIF-C OF评分(OR=2.275,95%CI:1.150~4.502,P<0.001)为HBV-ACLF短期预后的独立影响因素。代谢相关因素的联合预测模型AUC较单一预测模型增大,其中以高血压+TG+CLIF-C OF评分预测模型的AUC最大(AUC=0.886),合并代谢相关因素的患者肝脏相关并发症发生率更高,且30天病死率及90天病死率更高。 结论 高血压和血脂异常等代谢相关因素的存在会增加HBV-ACLF的严重程度并增加其短期死亡风险,高血压+TG+CLIF-C OF评分预测模型对HBV-ACLF患者短期预后有较好的预测价值。 Abstract:Objective To investigate the influence of metabolism-related factors (overweight and/or obesity, hyperglycemia, hypertension and dyslipidemia)on the 90-day prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF), and to establish a predictive model. Methods A retrospective analysis was performed for the clinical data of 365 patients with HBV-ACLF who were hospitalized in Department of Infectious Diseases, The Affiliated Hospital of Southwest Medical University, from June 2018 to June 2022, and according to the 90-day follow-up results, they were divided into survival group with 273 patients and death group with 92 patients. General information and related laboratory markers were collected from all patients. The t-test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distribution continuous data between groups; the chi-square test was used for comparison of categorical data between groups. A Logistic regression analysis was used to determine whether metabolism-related factors were independent risk factors for the 90-day prognosis of HBV-ACLF patients, and the Kaplan-Meier analysis was used to investigate the correlation between metabolism-related factors and the 90-day survival rate of HBV-ALCF patients. The area under the ROC curve (AUC) was used to compare the value of different scoring models in predicting the 90-day prognosis of HBV-ACLF patients. Results The multivariate Logistic regression analysis showed that hypertension (odds ratio [OR]=4.698, 95% confidence interval [CI]: 1.904 — 11.593, P=0.001), alanine aminotransferase (OR=0.999, 95%CI: 0.999 — 1.000, P=0.010), triglyceride (TG) (OR=4.979, 95%CI: 2.433 — 10.189, P<0.001), high-density lipoprotein cholesterol (OR=0.258, 95%CI: 0.087 — 0.762, P=0.012), apolipoprotein B (OR=0.118, 95%CI: 0.026 — 0.547, P=0.006), and CLIF-C OF score (OR=2.275, 95%CI: 1.150 — 4.502, P<0.001) were independent influencing factors for the short-term prognosis of HBV-ACLF. The combined predictive model of metabolism-related factors had a larger AUC than the predictive model of a single factor, among which the predictive model of hypertension+TG+CLIF-C OF score had the largest AUC of 0.886. The patients with metabolism-related factors tended to have higher incidence rate of liver complications and 30- and 90-day mortality rates. Conclusion The presence of the metabolism-related factors such as hypertension and dyslipidemia can increase the severity of HBV-ACLF and the risk of short-term mortality, and the hypertension+TG+CLIF-C OF score predictive model has a good value in predicting the short-term prognosis of HBV-ACLF patients. -
Key words:
- Hepatitis B virus /
- Acute-On-Chronic Liver Failure /
- Metabolism /
- Prognosis
-
表 1 CLIF-C OF评分
Table 1. CLIF-C OF score
项目 1分 2分 3分 TBil(mg/dL) <6 6~12 >12 Cr(mg/dL) <2 2~3.5 ≥3.5或肾脏替代 肝性脑病分级 0 Ⅰ~Ⅱ Ⅲ~Ⅳ INR <2 2~2.5 ≥2.5 平均动脉压(mmHg) ≥70 <70 应用血管活性药物 SPO2/FiO2 >357 215~357 ≤214 注:SPO2/FiO2,血氧饱和度/氧浓度。
表 2 生存组和死亡组基线特征的比较
Table 2. Comparison of baseline characteristics between the survivor group and death group
指标 生存组(n=273) 死亡组(n=92) 统计值 P值 年龄(岁) 49.85±12.35 53.96±11.35 t=-2.279 0.023 男/女(例) 217/56 77/15 χ2=0.778 0.378 BMI(kg/m²) 22.83(20.77~24.98) 23.88(21.86~25.88) Z=-2.524 0.012 合并糖尿病[例(%)] 87(31.9) 38(41.3) χ2=2.721 0.099 合并高血压[例(%)] 21(7.7) 27(29.3) χ2=28.254 <0.001 白细胞(×109/L) 6.41(4.90~8.42) 7.26(5.84~9.71) Z=-2.587 0.010 中性粒细胞(×109/L) 4.38(3.21~6.19) 5.49(4.04~7.91) Z=-3.541 <0.001 血红蛋白(g/L) 132.00(117.00~147.00) 129.00(111.00~142.75) Z=-2.115 0.034 红细胞(×1012/L) 4.20±0.79 4.04±0.86 t=1.592 0.112 红细胞压积(%) 0.39(0.34~0.43) 0.38(0.34~0.42) Z=-1.583 0.113 血小板(×109/L) 110.00(74.00~153.50) 94.50(65.25~129.00) Z=-2.242 0.025 ALT(U/L) 762.30(230.70~1 406.15) 463.35(168.98~1 047.35) Z=-2.643 0.008 AST(U/L) 510.50(200.05~1 090.74) 359.15(186.03~954.98) Z=-1.285 0.199 白蛋白(g/L) 33.03±4.77 30.65±4.79 t=4.133 <0.001 总蛋白(g/L) 67.20(62.60~72.40) 66.45(61.43~70.40) Z=-1.187 0.235 前白蛋白(g/L) 53.40(34.80~71.30) 42.55(26.45~63.85) Z=-2.697 0.007 DBil(μmol/L) 235.40(150.35~350.50) 313.30(235.33~449.28) Z=-4.760 <0.001 TBil(μmol/L) 173.50(102.10~254.85) 223.25(159.48~304.20) Z=-4.041 <0.001 总胆汁酸(μmol/L) 202.21±84.72 210.44±74.89 t=-0.828 0.408 GGT(U/L) 114.90(83.05~185.70) 104.10(72.30~170.18) Z=-1.376 0.169 ALP(U/L) 152.00(120.25~187.35) 150.15(118.58~189.88) Z=-0.055 0.956 Cr(μmol/L) 65.90(56.80~75.90) 67.70(54.88~87.00) Z=-1.281 0.200 肾小球滤过率(mL/min) 107.90(94.60~119.25) 102.60(77.15~115.70) Z=-2.218 0.027 血钾(mmol/L) 3.94±0.51 3.98±0.64 t=-0.574 0.567 血钠(mmol/L) 139.00(136.60~141.50) 136.30(133.58~139.95) Z=-4.890 <0.001 凝血酶原时间(s) 20.70(18.35~23.85) 25.85(21.65~32.58) Z=-6.986 <0.001 凝血酶原时间活动度(%) 44.00(36.00~54.00) 32.50(23.00~42.00) Z=-6.745 <0.001 INR 1.77(1.51~2.11) 2.33(1.89~3.17) Z=-7.010 <0.001 降钙素原(ng/mL) 0.92(0.57~1.54) 1.05(0.67~2.31) Z=-1.969 0.049 总胆固醇(mmol/L) 2.68(1.98~3.62) 2.79(2.14~3.85) Z=-1.369 0.171 TG(mmol/L) 1.32(0.98~1.70) 1.62(1.20~2.30) Z=-3.932 <0.001 HDL-C(mmol/L) 0.45(0.23~0.99) 0.22(0.07~0.45) Z=-5.944 <0.001 LDL-C(mmol/L) 1.53(1.17~2.03) 1.31(1.00~1.86) Z=-2.474 0.013 载脂蛋白B(g/L) 0.77(0.59~1.03) 0.68(0.54~0.93) Z=-2.525 0.012 载脂蛋白A1(g/L) 0.49(0.34~0.70) 0.40(0.19~0.57) Z=-3.578 <0.001 MELD-Na评分 23.19(20.50~26.59) 28.86(25.32~32.79) Z=-7.799 <0.001 CLIF-C OF评分 8(7~9) 10(9~12) Z=-9.655 <0.001 COSSH-ACLF评分 4.89(4.53~5.33) 6.05(5.35~6.93) Z=-9.309 <0.001 表 3 HBV-ACLF患者预后的Logistic回归分析
Table 3. Logistic regression analysis of prognosis in HBV-ACLF patients
因素 单因素分析 多因素分析 OR(95%CI) P值 OR(95%CI) P值 年龄 1.022(1.003~1.042) 0.024 BMI 1.092(1.014~1.177) 0.021 高血压 4.985(2.649~9.379) <0.001 4.698(1.904~11.593) 0.001 白细胞 1.075(1.003~1.152) 0.041 血红蛋白 0.987(0.976~0.998) 0.018 血小板 0.995(0.990~1.000) 0.032 白蛋白 0.901(0.855~0.949) 0.001 TBil 1.004(1.002~1.006) <0.001 ALT 0.999(0.999~1.000) 0.002 0.999(0.999~1.000) 0.010 肾小球滤过率 0.989(0.980~0.998) 0.017 凝血酶原时间活动度 0.939(0.920~0.958) <0.001 INR 3.063(2.152~4.361) <0.001 TG 2.100(1.515~2.910) <0.001 4.979(2.433~10.189) <0.001 HDL-C 0.117(0.053~0.259) <0.001 0.258(0.087~0.762) 0.012 LDL-C 0.646(0.445~0.938) 0.022 载脂蛋白B 0.194(0.075~0.499) 0.001 0.118(0.026~0.547) 0.006 载脂蛋白A1 0.326(0.145~0.735) 0.007 MELD-Na评分 1.126(1.083~1.171) <0.001 CLIF-C OF评分 2.226(1.849~2.680) <0.001 2.275(1.150~4.502) <0.001 COSSH-ACLF评分 3.545(2.581~4.869) <0.001 表 4 各模型预测HBV-ACLF患者90天病死率的效能分析
Table 4. Efficacy analysis of various models in predicting 90 day mortality rate of HBV-ACLF patients
评分模型 AUC 95%CI P值 cut-off值 敏感度 特异度 高血压 0.608 0.537~0.680 0.002 0.5 0.293 0.923 TG 0.637 0.569~0.705 <0.001 1.575 0.554 0.700 MELD-Na评分 0.772 0.718~0.826 <0.001 25.027 6 0.804 0.667 CLIF-C OF评分 0.830 0.784~0.875 <0.001 9.5 0.674 0.835 COSSH-ACLF评分 0.824 0.777~0.872 <0.001 5.433 4 0.750 0.773 高血压+TG+MELD-Na评分 0.831 0.785~0.877 <0.001 0.220 87 0.772 0.762 高血压+TG+CLIF-C OF评分 0.886 0.849~0.923 <0.001 0.332 412 0.750 0.872 高血压+TG+COSSH-ACLF评分 0.868 0.825~0.911 <0.001 0.188 104 0.837 0.758 注:AUC,ROC曲线下面积。
表 5 HBV-ACLF患者非MS组和MS组临床资料比较
Table 5. Comparison of clinical data of HBV-ACLF patients between non MS group and MS group
指标 非MS组(n=277) MS组(n=88) 统计值 P值 年龄(岁) 49.49±12.13 55.44±12.76 t=-3.419 0.001 男[例(%)] 225(81.23) 69(78.41) χ2=0.339 0.561 住院时间(天) 16.00(12.00~22.00) 15.00(10.25~19.00) Z=-2.071 0.038 30天病死率[例(%)] 41(14.80) 28(31.82) χ2=12.614 <0.001 90天病死率[例(%)] 57(20.58) 35(39.77) χ2=13.052 <0.001 并发症[例(%)] 细菌感染 159(57.40) 63(71.59) χ2=5.643 0.018 肝硬化 157(56.68) 61(69.32) χ2=4.435 0.035 腹水[例(%)] χ2=-0.721 0.471 无 136(49.10) 40(45.45) 1级 54(19.49) 17(19.32) 2级 53(19.13) 18(20.45) 3级 34(12.27) 13(14.77) 肝性脑病[例(%)] χ2=-1.163 0.048 0期 229(82.67) 69(78.41) 1期 10(3.61) 3(3.41) 2期 18(6.50) 2(2.27) 3期 14(5.05) 7(7.95) 4期 6(2.17) 7(7.95) 白细胞(×109/L) 6.44(4.94~8.48) 7.11(5.43~9.50) Z=-1.982 0.047 血红蛋白(g/L) 132(115~146) 132(117~144) Z=-0.176 0.861 血小板(×109/L) 107.00(71.50~145.00) 102.00(72.25~148.50) Z=-0.249 0.804 ALT(U/L) 692.30(204.80~1 347.75) 577.00(248.45~1 209.53) Z=-0.473 0.636 AST(U/L) 504.20(190.25~1 054.60) 435.80(211.83~1 044.88) Z=-0.263 0.793 白蛋白(g/L) 32.70±5.02 31.59±4.32 t=1.873 0.620 TBil(μmol/L) 251.30(163.40~373.45) 264.25(176.90~370.25) Z=-0.510 0.610 Cr(μmol/L) 65.80(54.75~76.55) 69.65(61.33~83.65) Z=-2.565 0.010 肾小球滤过率(mL/min) 108.50(96.90~120.25) 98.50(81.08~113.85) Z=-3.800 <0.001 血清钠(mmol/L) 137.60(135.20~139.75) 137.20(135.43~138.90) Z=-0.712 0.476 凝血酶原时间(s) 21.00(18.40~25.45) 22.35(20.00~28.60) Z=-2.868 0.004 凝血酶原活动度(%) 43.44±15.21 38.13±13.05 t=2.949 0.003 INR 1.83(1.51~2.29) 1.97(1.70~2.64) Z=-2.849 0.004 MELD-Na评分 24.21(21.13~28.04) 25.06(22.29~30.31) Z=-2.040 0.041 CLIF-C OF评分 8(8~10) 9(8~10) Z=-2.141 0.032 COSSH-ACLF评分 4.50(4.56~5.71) 5.51(4.97~6.20) Z=-4.114 <0.001 -
[1] Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Guideline for diagnosis and treatment of liver failure(2018)[J]. J Clin Hepatol, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007.中华医学会感染病学分会肝衰竭与人工肝学组, 中华医学会肝病学分会重型肝病与人工肝学组. 肝衰竭诊治指南(2018年版)[J]. 临床肝胆病杂志, 2019, 35( 1): 38- 44. DOI: 10.3969/j.issn.1001-5256.2019.01.007. [2] CHEN MJ, LI X, TANG SH. Advances in multi-dimensional assessment of liver function in the prognosis of patients with liver failure[J]. Clin J Med Offic, 2023, 51( 9): 901- 903, 907. DOI: 10.16680/j.1671-3826.2023.09.05.陈美娟, 李雪, 汤善宏. 多维度评估肝功能在肝衰竭患者预后中研究进展[J]. 临床军医杂志, 2023, 51( 9): 901- 903, 907. DOI: 10.16680/j.1671-3826.2023.09.05. [3] ASRANI S, SIMONETTO D, KAMATH P. Acute-on-chronic liver failure[J]. Clin Gastroenterol Hepatol, 2015, 13( 12): 2128- 39. DOI: 10.1016/j.cgh.2015.07.008. [4] MEZZANO G, JUANOLA A, CARDENAS A, et al. Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis[J]. Gut, 2022, 71( 1): 148- 155. DOI: 10.1136/gutjnl-2020-322161. [5] BERZIGOTTI A, GARCIA-TSAO G, BOSCH J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis[J]. Hepatology, 2011, 54( 2): 555- 561. DOI: 10.1002/hep.24418. [6] SARIN SK, CHOUDHURY A, SHARMA MK, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver(APASL): An update[J]. Hepatol Int, 2019, 13( 4): 353- 390. DOI: 10.1007/s12072-019-09946-3. [7] Chinese Society of Hepatology and Chinese Society of Infectious Disease, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B: 2019 version[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2019年版)[J]. 临床肝胆病杂志, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. [8] Metabolic Syndrome Research Collaboration Group, Diabetes Branch, Chinese Medical Association. Recommendations on metabolic syndrome, diabetes branch, Chinese Medical Association[J]. Chin J Diabetes, 2004, 12( 3): 5- 10.中华医学会糖尿病学分会代谢综合征研究协作组. 中华医学会糖尿病学分会关于代谢综合征的建议[J]. 中华糖尿病杂志, 2004, 12( 3): 5- 10. [9] FAN Q, LI Z. Liver transplantation for acute-on-chronic liver failure[J]. Organ Transplantation, 2022, 13( 3): 333- 337. DOI: 10.3969/j.issn.1674-7445.2022.03.008.范祺, 李照. 慢加急性肝衰竭的肝移植治疗[J]. 器官移植, 2022, 13( 3): 333- 337. DOI: 10.3969/j.issn.1674-7445.2022.03.008. [10] FAHED G, AOUN L, ZERDAN M BOU, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021[J]. Int J Mol Sci, 2022, 23( 2): 786. DOI: 10.3390/ijms23020786. [11] LAM DW, LEROITH D, FEINGOLD KR, et al. Metabolic syndrome[R]. Endotext. MDText.com, Inc, 2000. [12] HIRODE G, WONG RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016[J]. JAMA, 2020, 323( 24): 2526- 2528. DOI: 10.1001/jama.2020.4501. [13] SETO WK. Chronic hepatitis B and metabolic risk factors: A call for rigorous longitudinal studies[J]. World J Gastroenterol, 2019, 25( 3): 282- 286. DOI: 10.3748/wjg.v25.i3.282. [14] REN HN, WANG JN, GAO Y, et al. Metabolic syndrome and liver-related events: A systematic review and meta-analysis[J]. BMC Endocr Disord, 2019, 19( 1): 40. DOI: 10.1186/s12902-019-0366-3. [15] DIAO YT, HU DQ, HU X, et al. The role of metabolic factors and steatosis in treatment-Naïve patients with chronic hepatitis B and normal alanine aminotransferase[J]. Infect Dis Ther, 2022, 11( 3): 1133- 1148. DOI: 10.1007/s40121-022-00629-5. [16] DUSEJA A, DE A, TANEJA S, et al. Impact of metabolic risk factors on the severity and outcome of patients with alcohol-associated acute-on-chronic liver failure[J]. Liver Int, 2021, 41( 1): 150- 157. DOI: 10.1111/liv.14671. [17] ENGIN A. The pathogenesis of obesity-associated adipose tissue inflammation[J]. Adv Exp Med Biol, 2017, 960: 221- 245. DOI: 10.1007/978-3-319-48382-5_9. [18] PARADIS V, PERLEMUTER G, BONVOUST F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: A potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis[J]. Hepatology, 2001, 34( 4 Pt 1): 738- 744. DOI: 10.1053/jhep.2001.28055. [19] PERGOLA GD, PANNACCIULLI N. Coagulation and fibrinolysis abnormalities in obesity[J]. J Endocrinol Invest, 2002, 25( 10): 899- 904. DOI: 10.1007/BF03344054. [20] LAAT-KREMERS RD, CASTELNUOVO AD, van der VORM L, et al. Increased BMI and blood lipids are associated with a hypercoagulable state in the moli-sani cohort[J]. Front Cardiovasc Med, 2022, 9: 897733. DOI: 10.3389/fcvm.2022.897733. -



 PDF下载 ( 903 KB)
PDF下载 ( 903 KB)

 下载:
下载:



