华蟾素调控AKT介导的上皮间质转化抑制肝细胞癌肺转移裸鼠模型的作用机制
DOI: 10.12449/JCH240919
伦理学声明:本研究方案于2019年4月18日经由上海中医药大学附属龙华医院实验动物伦理委员会审批,批号:LHERAW-19017,符合实验室动物管理与使用准则。
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:杨悦、续嗣钰、王珏、杜施霖参与收集数据,资料分析;杨悦负责撰写论文;张春蕾、宋海燕负责课题设计,指导撰写文章并最后定稿。
Mechanism of action of cinobufotalin in inhibiting lung metastasis of hepatocellular carcinoma by regulating AKT-mediated epithelial-mesenchymal transition in a nude mouse model
-
摘要:
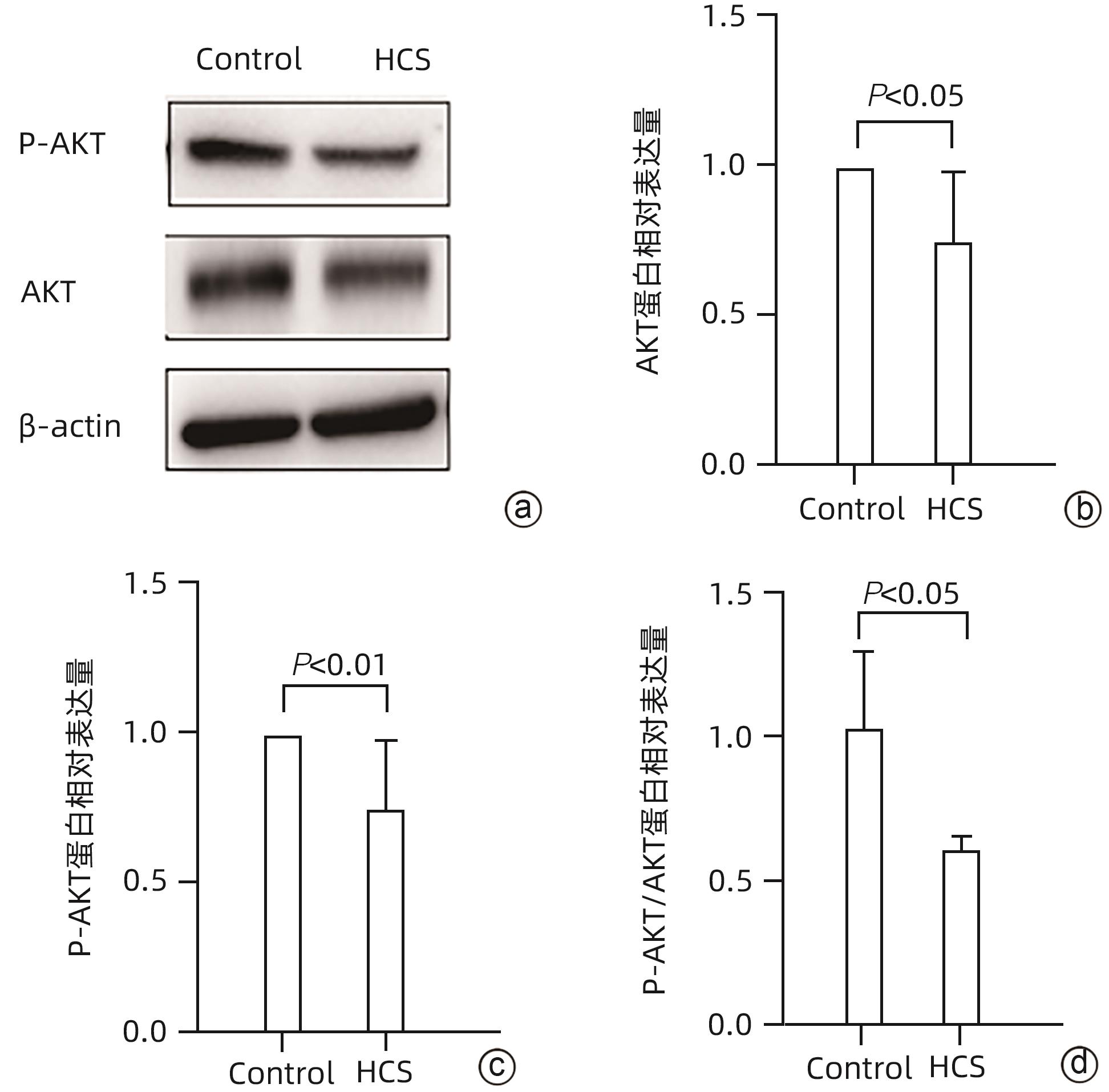
目的 研究华蟾素通过调控肝细胞癌(HCC)上皮间质转化(EMT)抑制HCC转移的作用和机制。 方法 将36只6周龄雄性BALB/c裸鼠尾静脉注射MHCC97H细胞建立肝癌肺转移瘤模型,随机分为华蟾素高、低剂量组和对照组。建模当日起分别腹腔注射华蟾素120 μL/kg、60 μL/kg或生理盐水,每周2次。8周后取肺组织行HE染色检测肝癌肺转移率。MHCC97H细胞用华蟾素高、低剂量(2.5 μL/mL、5 μL/mL)干预,通过划痕实验、RT-PCR以及Western Blot检测细胞迁移能力和EMT相关分子的表达。用CoCl2孵育模拟低氧环境诱导MHCC97H细胞,同时加入高、低剂量华蟾素干预,通过划痕实验和Western Blot检测华蟾素对低氧诱导的细胞迁移能力和EMT的影响。使用转录组学分析华蟾素对MHCC97H细胞的效应机制。用Western Blot检验华蟾素干预对MHCC97H细胞的蛋白激酶B(AKT)、磷酸化AKT(P-AKT)表达水平的影响。计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验,两组间比较采用成组t检验。 结果 华蟾素干预组裸鼠较对照组肝癌肺转移率下降。与对照组相比,华蟾素干预使MHCC97H细胞划痕愈合率减小、上皮型分子表达上调(t=2.860,P<0.05),并使EMT转录因子和基质型分子下调(t值分别为3.545、2.022、2.852、2.341,P值均<0.05)。低氧诱导上调MHCC97H细胞划痕愈合率和基质型分子、EMT转录因子表达水平(P值均<0.05),华蟾素干预逆转EMT变化并抑制划痕愈合(P值均<0.05)。肝癌细胞转录组学分析显示,华蟾素组与对照组存在显著的基因差异,华蟾素主要影响了肿瘤、代谢、免疫和信号传导相关基因表达,其中AKT信号转导通路中的差异基因数量最多。进一步检测发现华蟾素干预可下调HCC 细胞AKT、P-AKT和P-AKT/AKT的水平(t值分别为2.434、3.401、2.258,P值均<0.05)。 结论 华蟾素可抑制肝癌转移,尤其对于低氧环境诱导的肝癌转移具有显著抑制作用,调控AKT信号转导通路介导的HCC细胞EMT可能是其部分作用机制。 Abstract:Objective To investigate the effect and mechanism of cinobufotalin in inhibiting hepatocellular carcinoma (HCC) metastasis by regulating epithelial-mesenchymal transition (EMT). Methods A total of 36 male BALB/c nude mice, aged 6 weeks, were given injection of MHCC97H cells via the caudal vein to establish a model of HCC lung metastasis, and then the mice were randomly divided into high-and low-dose cinobufotalin groups and control group. Since the day of modeling, the mice in the high-and low-dose cinobufotalin groups were given intraperitoneal injection of cinobufotalin at a dose of 120 μL/kg and 60 μL/kg, respectively, and those in the control group were given intraperitoneal injection of normal saline, twice a week. After 8 weeks, HE staining was performed for lung tissue to measure the lung metastasis rate of HCC. MHCC97H cells were treated with high-dose (2.5 μL/mL) or low-dose (5 μL/mL) cinobufotalin for 24 hours, and wound healing assay, RT-PCR, and Western blot were used to measure cell migration ability and the expression of EMT-related molecules. MHCC97H cells were induced in a simulated hypoxic environment with CoCl2 incubation, with high- and low-dose cinobufotalin added for intervention, and wound healing assay and Western blot were used to investigate the effect of cinobufotalin on cell migration ability and EMT induced by hypoxia. Transcriptome analysis was used to investigate the effect mechanism of cinobufotalin on MHCC97H cells, and Western blot was used to observe the effect of cinobufotalin on the expression levels of protein kinase B (AKT) and phosphorylated AKT (P-AKT) in MHCC97H cells. A one-way analysis of variance was used for comparison of continuous data between multiple groups, and the least significant difference t-test was used for further comparison between two groups; the independent-samples t test was used for comparison of categorical data between two groups. Results Compared with the control group, the cinobufotalin group had a significant reduction in the lung metastasis rate of HCC. Compared with the control group, cinobufotalin intervention reduced the wound healing rate of MHCC97H cells, upregulated the expression of epithelial-type molecules (t=2.860, P<0.05), and downregulated the expression of EMT transcription factors (EMT-TFs) and mesenchymal molecules (t=3.545, 2.022, 2.852, and 2.341, all P<0.05). Hypoxia induction upregulated the wound healing rate of MHCC97H cells and the expression levels of mesenchymal molecules and EMT-TFs (P<0.05), and cinobufotalin intervention reversed EMT change and inhibited wound healing (P<0.05). The transcriptome analysis of MHCC97H cells showed significant gene differences between the cinobufotalin group and the control group, and cinobufotalin mainly affected the expression of genes associated with tumor, metabolism, immunity, and signal transduction, with the largest number of differentially expressed genes in the AKT signal transduction pathway. Further measurement showed that cinobufotalin intervention downregulated the expression levels of AKT, P-AKT, and P-AKT/AKT in MHCC97H cells (t=2.434, 3.401, and 2.258, all P<0.05). Conclusion Cinobufotalin can inhibit the metastasis of HCC, especially hypoxia-induced HCC metastasis, and regulation of EMT mediated by the AKT signal transduction pathway in HCC cells might be one of its mechanisms of action. -
-
[1] Prospective suRveillance for very Early hepatoCellular cARcinoma(PreCar) expert panel. Expert consensus on early screening strategies for liver cancer in China[J]. Chin Hepatol, 2021, 26( 8): 825- 831. DOI: 10.3969/j.issn.1008-1704.2021.08.001.全国多中心前瞻性肝癌极早期预警筛查项目(PreCar)专家组. 中国肝癌早筛策略专家共识[J]. 肝脏, 2021, 26( 8): 825- 831. DOI: 10.3969/j.issn.1008-1704.2021.08.001. [2] KONYN P, AHMED A, KIM D. Current epidemiology in hepatocellular carcinoma[J]. Expert Rev Gastroenterol Hepatol, 2021, 15( 11): 1295- 1307. DOI: 10.1080/17474124.2021.1991792. [3] CHIN KM, ALLEN JC, TEO JY, et al. Predictors of post-hepatectomy liver failure in patients undergoing extensive liver resections for hepatocellular carcinoma[J]. Ann Hepatobiliary Pancreat Surg, 2018, 22( 3): 185- 196. DOI: 10.14701/ahbps.2018.22.3.185. [4] ZHANG YT, LI BP, ZHANG B, et al. LncRNA SBF2-AS1 promotes hepatocellular carcinoma metastasis by regulating EMT and predicts unfavorable prognosis[J]. Eur Rev Med Pharmacol Sci, 2018, 22( 19): 6333- 6341. DOI: 10.26355/eurrev_201810_16044. [5] PASTUSHENKO I, BLANPAIN C. EMT transition states during tumor progression and metastasis[J]. Trends Cell Biol, 2019, 29( 3): 212- 226. DOI: 10.1016/j.tcb.2018.12.001. [6] ZHANG G, ZHANG GY. Upregulation of FoxP4 in HCC promotes migration and invasion through regulation of EMT[J]. Oncol Lett, 2019, 17( 4): 3944- 3951. DOI: 10.3892/ol.2019.10049. [7] HUANG YH, HONG WQ, WEI XW. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis[J]. J Hematol Oncol, 2022, 15( 1): 129. DOI: 10.1186/s13045-022-01347-8. [8] SHEN JJ. The clinical effect of Cinobufagin injection by transcatheter arterial chemoembolization(TACE) combined with intravenous on treating primary liver cancer(PLC)[J]. J Clin Hepatol, 2009, 25( 3): 207- 209. DOI: 10.3969/j.issn.1001-5256.2009.03.018.沈建军. 华蟾素介入栓塞联合静脉注射对原发性肝癌的临床疗效[J]. 临床肝胆病杂志, 2009, 25( 3): 207- 209. DOI: 10.3969/j.issn.1001-5256.2009.03.018. [9] YU T, ZHAI XF, LIU Q, et al. Clinical effect and safety of hepatic arterial infusion of cinobufotalin injection combined with lipiodol chemoembolization in treatment of Barcelona Clinic Liver Cancer stage C primary liver cancer[J]. J Clin Hepatol, 2017, 33( 2): 281- 285. DOI: 10.3969/j.issn.1001-5256.2017.02.015.于童, 翟笑枫, 刘群, 等. 华蟾素注射液经肝动脉灌注联合碘油栓塞治疗巴塞罗那C期原发性肝癌的效果和安全性[J]. 临床肝胆病杂志, 2017, 33( 2): 281- 285. DOI: 10.3969/j.issn.1001-5256.2017.02.015. [10] CHENG L, CHEN YZ, PENG Y, et al. Ceramide production mediates cinobufotalin-induced growth inhibition and apoptosis in cultured hepatocellular carcinoma cells[J]. Tumour Biol, 2015, 36( 8): 5763- 5771. DOI: 10.1007/s13277-015-3245-1. [11] LI WQ, PEI SH, ZHANG XJ, et al. Cinobufotalin inhibits the epithelial-mesenchymal transition of hepatocellular carcinoma cells through down-regulate β-catenin in vitro and in vivo[J]. Eur J Pharmacol, 2022, 922: 174886. DOI: 10.1016/j.ejphar.2022.174886. [12] YANG SY, ZHANG JJ, HUANG XY. Mouse models for tumor metastasis[J]. Methods Mol Biol, 2012, 928: 221- 228. DOI: 10.1007/978-1-62703-008-3_17. [13] ZHU L, CHEN YX, WEI C, et al. Anti-proliferative and pro-apoptotic effects of cinobufagin on human breast cancer MCF-7 cells and its molecular mechanism[J]. Nat Prod Res, 2018, 32( 4): 493- 497. DOI: 10.1080/14786419.2017.1315575. [14] YUAN ZT, SHI XJ, QIU YY, et al. Reversal of P-gp-mediated multidrug resistance in colon cancer by cinobufagin[J]. Oncol Rep, 2017, 37( 3): 1815- 1825. DOI: 10.3892/or.2017.5410. [15] GUO N, MIAO YY, SUN MZ. Transcatheter hepatic arterial chemoembolization plus cinobufotalin injection adjuvant therapy for advanced hepatocellular carcinoma: A meta-analysis of 27 trials involving 2, 079 patients[J]. Onco Targets Ther, 2018, 11: 8835- 8853. DOI: 10.2147/OTT.S182840. [16] CHEN Z, CHEN HY, LANG QB, et al. Preventive effects of Jiedu Granules combined with cinobufacini injection versus transcatheter arterial chemoembolization in post-surgical patients with hepatocellular carcinoma: A case-control trial[J]. Chin J Integr Med, 2012, 18( 5): 339- 344. DOI: 10.1007/s11655-012-1083-1. [17] WEI YH, YANG CX, YANG GM, et al. Inhibitory effect of downregulating HMGB2 expression on epithelial-mesenchymal transition of liver cancer LM3 cells and its AKT/m TOR signaling pathway mechanism[J]. J Jilin Univ Med Ed, 2024, 50( 1): 143- 149. DOI: 10.13481/j.1671-587X.20240118.魏雁虹, 杨晨雪, 杨广民, 等. 下调HMGB2表达对肝癌LM3细胞上皮-间质转化的抑制作用及其AKT/mTOR信号通路机制[J]. 吉林大学学报(医学版), 2024, 50( 1): 143- 149. DOI: 10.13481/j.1671-587X.20240118. [18] HAY ED. An overview of epithelio-mesenchymal transformation[J]. Acta Anat, 1995, 154( 1): 8- 20. DOI: 10.1159/000147748. [19] DUDAS J, LADANYI A, INGRUBER J, et al. Epithelial to mesenchymal transition: A mechanism that fuels cancer radio/chemoresistance[J]. Cells, 2020, 9( 2): 428. DOI: 10.3390/cells9020428. [20] ZHANG Y, WEINBERG RA. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities[J]. Front Med, 2018, 12( 4): 361- 373. DOI: 10.1007/s11684-018-0656-6. [21] AIELLO NM, MADDIPATI R, NORGARD RJ, et al. EMT subtype influences epithelial plasticity and mode of cell migration[J]. Dev Cell, 2018, 45( 6): 681- 695. e 4. DOI: 10.1016/j.devcel.2018.05.027. [22] VAUPEL P, MAYER A, HÖCKEL M. Tumor hypoxia and malignant progression[J]. Methods Enzymol, 2004, 381: 335- 354. DOI: 10.1016/S0076-6879(04)81023-1. [23] VAUPEL P, HÖCKEL M, MAYER A. Detection and characterization of tumor hypoxia using PO2 histography[J]. Antioxid Redox Signal, 2007, 9( 8): 1221- 1235. DOI: 10.1089/ars.2007.1628. [24] LIU ZK, WANG YF, DOU CW, et al. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma[J]. Theranostics, 2018, 8( 17): 4649- 4663. DOI: 10.7150/thno.26789. [25] JU SW, WANG F, WANG YR, et al. CSN8 is a key regulator in hypoxia-induced epithelial-mesenchymal transition and dormancy of colorectal cancer cells[J]. Mol Cancer, 2020, 19( 1): 168. DOI: 10.1186/s12943-020-01285-4. [26] CORTI F, NICHETTI F, RAIMONDI A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives[J]. Cancer Treat Rev, 2019, 72: 45- 55. DOI: 10.1016/j.ctrv.2018.11.001. [27] LUO H, YU YY, CHEN HM, et al. The combination of NVP-BEZ235 and rapamycin regulates nasopharyngeal carcinoma cell viability and apoptosis via the PI3K/AKT/mTOR pathway[J]. Exp Ther Med, 2019, 17( 1): 99- 106. DOI: 10.3892/etm.2018.6896. [28] WU DM, ZHANG T, LIU YB, et al. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling[J]. Cell Death Dis, 2019, 10( 5): 349. DOI: 10.1038/s41419-019-1591-4. [29] LIU M, HUANG XD, HAN Z, et al. Effect of cadherin-17 on proliferation and apoptosis of colorectal cancer cells and its PI3K/AKT/mTOR signaling pathway regulatory mechanism[J]. J Jilin Univ(Med Ed), 2023, 49( 4): 1008- 1017. DOI: 10.13481/j.1671-587X.20230423.刘蒙, 黄晓东, 韩峥, 等. 钙黏蛋白17对结直肠癌细胞增殖和凋亡的影响及其PI3K/AKT/mTOR信号通路调节机制[J]. 吉林大学学报(医学版), 2023, 49( 4): 1008- 1017. DOI: 10.13481/j.1671-587X.20230423. [30] XU T, ZHANG R, DONG ML, et al. Osteoglycin(OGN) inhibits cell proliferation and invasiveness in breast cancer via PI3K/akt/mTOR signaling pathway[J]. Onco Targets Ther, 2019, 12: 10639- 10650. DOI: 10.2147/OTT.S222967. [31] YEH YH, WANG SW, YEH YC, et al. Rhapontigenin inhibits TGF-β-mediated epithelial-mesenchymal transition via the PI3K/AKT/mTOR pathway and is not associated with HIF-1α degradation[J]. Oncol Rep, 2016, 35( 5): 2887- 2895. DOI: 10.3892/or.2016.4664. [32] LI CY, WANG Q, WANG XM, et al. Scutellarin inhibits the invasive potential of malignant melanoma cells through the suppression epithelial-mesenchymal transition and angiogenesis via the PI3K/Akt/mTOR signaling pathway[J]. Eur J Pharmacol, 2019, 858: 172463. DOI: 10.1016/j.ejphar.2019.172463. [33] WANG HY, ZHANG CY, XU LT, et al. Bufalin suppresses hepatocellular carcinoma invasion and metastasis by targeting HIF-1α via the PI3K/AKT/mTOR pathway[J]. Oncotarget, 2016, 7( 15): 20193- 20208. DOI: 10.18632/oncotarget.7935. [34] XING SP, YU WX, ZHANG XF, et al. Isoviolanthin extracted from Dendrobium officinale reverses TGF-β1-mediated Epithelial⁻Mesenchymal transition in hepatocellular carcinoma cells via deactivating the TGF-β/smad and PI3K/akt/mTOR signaling pathways[J]. Int J Mol Sci, 2018, 19( 6): 1556. DOI: 10.3390/ijms19061556. [35] GHALALI A, YE ZW, HÖGBERG J, et al. PTEN and PHLPP crosstalk in cancer cells and in TGFβ-activated stem cells[J]. Biomed Pharmacother, 2020, 127: 110112. DOI: 10.1016/j.biopha.2020.110112. -



 PDF下载 ( 3425 KB)
PDF下载 ( 3425 KB)


 下载:
下载: