大道至简——简约慢性乙型肝炎疾病进展自然史建议
DOI: 10.12449/JCH240905
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:刘新负责撰写论文;李嘉负责修改论文;鲁凤民负责拟定写作思路,指导论文撰写并修改论文。
The way is simple: A brief natural history of chronic hepatitis B virus infection and disease progression
-
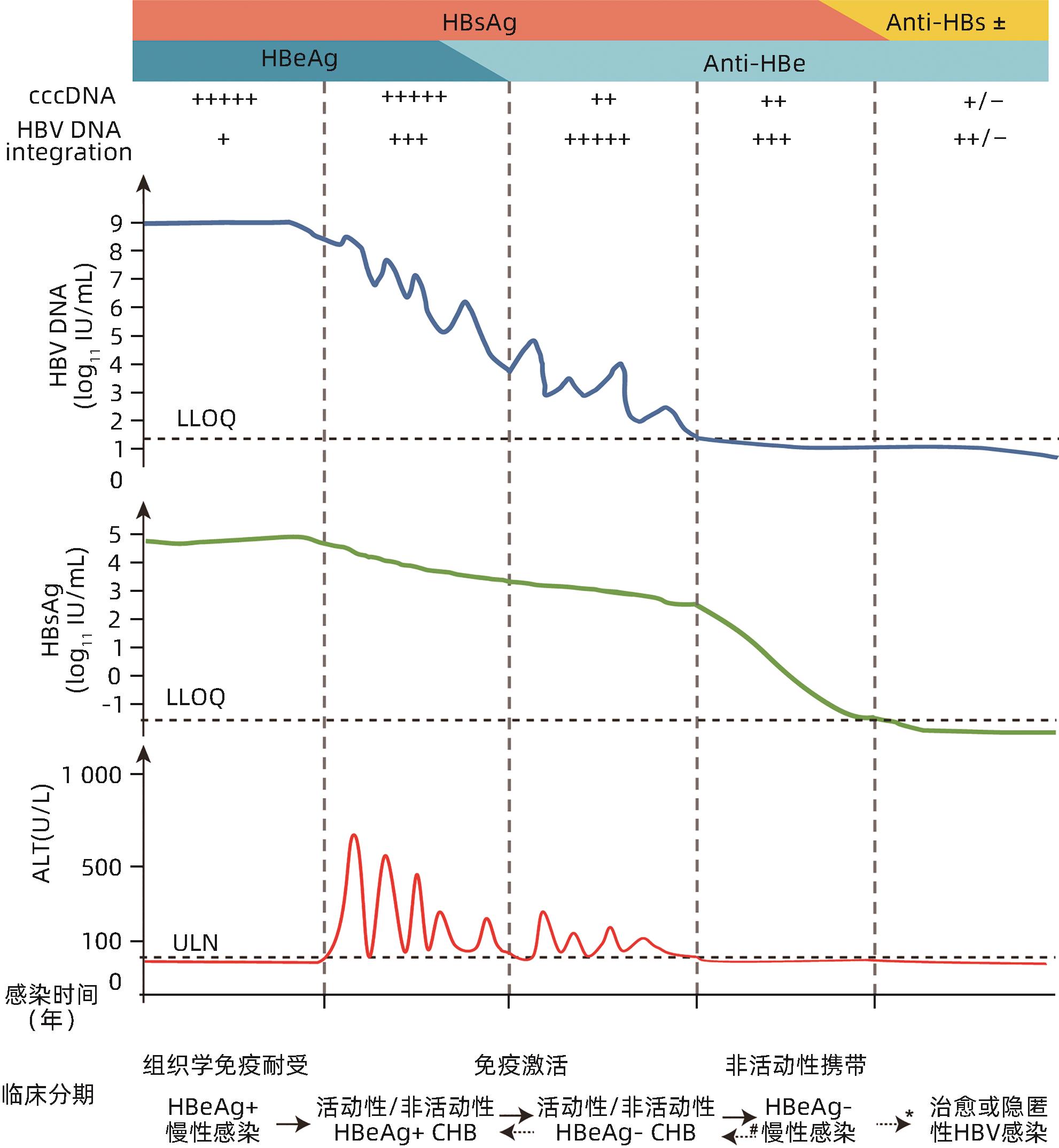
摘要: 慢性HBV感染是慢性乙型肝炎、肝硬化和原发性肝癌的主要病因,为社会造成巨大的卫生和经济负担。慢性乙型肝炎的疾病进展由病毒、宿主免疫及感染肝细胞的相互作用所共同驱动。慢性HBV感染自然史分期的意义在于理解疾病进程、评估疾病进展阶段,指导抗病毒治疗时机及方案选择。或许是因为对是否有真正的免疫耐受期存在争议,我国《慢性乙型肝炎防治指南(2022年版)》淡化了疾病状态与宿主免疫状态的对应关系,对慢性HBV感染自然史的更新描述采用了2017版欧洲肝病学会指南的4种疾病阶段:HBeAg阳性慢性HBV感染、HBeAg阳性慢性乙型肝炎、HBeAg阴性慢性HBV感染和HBeAg阴性慢性乙型肝炎。同时,新指南也未能完全解决“不确定期”的问题。随着临床上扩大抗病毒治疗策略渐成趋势,现有的自然史分期已难以适应临床需要,更新势在必行。本文从慢性HBV感染自然史的发现历程以及现有自然史分期存在的问题和建议等方面展开阐述,以期简化自然史分期,适应现有抗病毒治疗方案的临床实践,方便临床医生进行治疗决策。Abstract: Chronic hepatitis B virus (HBV) infection is the major cause of chronic hepatitis B (CHB), liver cirrhosis, and primary hepatocellular carcinoma (HCC) and brings huge health and economic burdens to the society. The disease progression of CHB is driven by the interaction between the virus, host immune response, and infected hepatocytes. Staging of the natural history of chronic HBV infection will help to understand disease progression, assess the stage of disease progression, and provide guidance for determining the time and regimen of antiviral therapy. Due to the controversy over the existence of a true immune tolerance phase, Guidelines for the prevention and treatment of chronic hepatitis B (2022 edition) in China weakens the association between disease state and host immune status and provides an updated description of the natural history of chronic HBV infection based on the four disease stages from the 2017 European Association for the Study of the Liver (EASL) guidelines, i.e., HBeAg-positive chronic HBV infection, HBeAg-positive CHB, HBeAg-negative chronic HBV infection, and HBeAg-negative CHB. Moreover, it fails to fully resolve the issue of the “indeterminate phase”. With the growing trend of expanding antiviral treatment strategies in clinical practice, the current staging system based on natural history can hardly meet clinical needs, and thus it is necessary to make updates. This article elaborates on the discovery of the natural history of chronic HBV infection, the problems of the existing staging system of natural history, and related recommendations, in order to simplify the staging system of natural history, align with current antiviral treatment regimens, and facilitate clinical decision-making by clinicians.
-
Key words:
- Hepatitis B virus /
- Infections /
- Immune Tolerance
-
[1] Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: A modelling study[J]. Lancet Gastroenterol Hepatol, 2023, 8( 10): 879- 907. DOI: 10.1016/S2468-1253(23)00197-8. [2] BLUMBERG BS. A“new” antigen in leukemia sera[J]. JAMA, 1965, 191( 7): 541. DOI: 10.1001/jama.1965.03080070025007. [3] REALDI G, ALBERTI A, RUGGE M, et al. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection[J]. Gastroenterology, 1980, 79( 2): 195- 199. [4] HOOFNAGLE JH, DUSHEIKO GM, SEEFF LB, et al. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis[J]. Ann Intern Med, 1981, 94( 6): 744- 748. DOI: 10.7326/0003-4819-94-6-744. [5] CHU CM, KARAYIANNIS P, FOWLER MJ, et al. Natural history of chronic hepatitis B virus infection in Taiwan: Studies of hepatitis B virus DNA in serum[J]. Hepatology, 1985, 5( 3): 431- 434. DOI: 10.1002/hep.1840050315. [6] YIM HJ, LOK ASF. Natural history of chronic hepatitis B virus infection: What we knew in 1981 and what we know in 2005[J]. Hepatology, 2006, 43(2 Suppl 1): S173- S181. DOI: 10.1002/hep.20956. [7] MASON WS, GILL US, LITWIN S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant[J]. Gastroenterology, 2016, 151( 5): 986- 998. e 4. DOI: 10.1053/j.gastro.2016.07.012. [8] European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67( 2): 370- 398. DOI: 10.1016/j.jhep.2017.03.021. [9] CHEN YC, CHU CM, LIAW YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B[J]. Hepatology, 2010, 51( 2): 435- 444. DOI: 10.1002/hep.23348. [10] MORITA S, MATSUMOTO A, UMEMURA T, et al. Characteristics and prediction of hepatitis B e-antigen negative hepatitis following seroconversion in patients with chronic hepatitis B[J]. Hepatol Res, 2014, 44( 10): E45- E53. DOI: 10.1111/hepr.12208. [11] JENG WJ, PAPATHEODORIDIS GV, LOK ASF. Hepatitis B[J]. Lancet, 2023, 401( 10381): 1039- 1052. DOI: 10.1016/s0140-6736(22)01468-4. [12] WANG LJ, LI MW, LIU YN, et al. Natural history and disease progression of chronic hepatitis B virus infection[J]. J Peking Univ Health Sci, 2022, 54( 5): 920- 926. DOI: 10.19723/j.issn.1671-167X.2022.05.019.王雷婕, 李明蔚, 刘燕娜, 等. 慢性乙型肝炎病毒感染的自然病程特征[J]. 北京大学学报(医学版), 2022, 54( 5): 920- 926. DOI: 10.19723/j.issn.1671-167X.2022.05.019. [13] Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Clin Infect Dis, 2022, 15( 6): 401- 427. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.001.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 中华临床感染病杂志, 2022, 15( 6): 401- 427. DOI: 10.3760/cma.j.issn.1674-2397.2022.06.001. [14] KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134( 5): 1376- 1384. DOI: 10.1053/j.gastro.2008.02.075. [15] HSU YN, PAN CQ, ABBASI A, et al. Clinical presentation and disease phases of chronic hepatitis B using conventional versus modified ALT criteria in Asian Americans[J]. Dig Dis Sci, 2014, 59( 4): 865- 871. DOI: 10.1007/s10620-014-3054-1. [16] XU XQ, WANG H, SHAN S, et al. The impact of the definitions of clinical phases on the profiles of grey-zone patients with chronic hepatitis B virus infection[J]. Viruses, 2023, 15( 5): 1212. DOI: 10.3390/v15051212. [17] GAN QY, WANG JX, QIAN F, et al. Clinical and histological features of patients with chronic hepatitis B virus infection in the grey zone[J]. J Viral Hepat, 2023, 30( 10): 803- 809. DOI: 10.1111/jvh.13873. [18] WANG J, YAN XM, ZHU L, et al. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone[J]. Aliment Pharmacol Ther, 2023, 57( 5): 464- 474. DOI: 10.1111/apt.17272. [19] CHOI GH, KIM GA, CHOI J, et al. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation[J]. Aliment Pharmacol Ther, 2019, 50( 2): 215- 226. DOI: 10.1111/apt.15311. [20] TENG W, CHANG TT, YANG HI, et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines[J]. Hepatol Int, 2021, 15( 6): 1421- 1430. DOI: 10.1007/s12072-021-10263-x. [21] HUANG DQ, TRAN A, YEH ML, et al. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase[J]. Hepatology, 2023, 78( 5): 1558- 1568. DOI: 10.1097/HEP.0000000000000459. [22] JIANG B, WANG LJ, LIU H, et al. Association of HBV serological markers with host antiviral immune response relevant hepatic inflammatory damage in chronic HBV infection[J]. J Med Virol, 2024, 96( 4): e29569. DOI: 10.1002/jmv.29569. [23] WANG LJ, WANG J, ZHAO KY, et al. The relationship between viral replication and the severity of hepatic necroinflammatory damage changed before HBeAg loss in patients with chronic hepatitis B virus infection[J]. J Clin Transl Hepatol, 2024, 12( 4): 381- 388. DOI: 10.14218/JCTH.2023.00378. [24] XING TJ, ZHAO KY, LI WT, et al. Association between HBV viral load and severity of liver inflammation in patients with chronic hepatitis B virus infection[J]. Chin J Hepatol, 2023, 31( 9): 954- 960. DOI: 10.3760/cma.j.cn501113-20230820-00061.邢同京, 赵坤宇, 李文涛, 等. 慢性HBV感染者病毒DNA水平与患者肝组织炎症损伤程度的相关性研究[J]. 中华肝脏病杂志, 2023, 31( 9): 954- 960. DOI: 10.3760/cma.j.cn501113-20230820-00061. [25] LIU X, WANG LJ, LU FM. A commentary on the natural disease progression sequence of chronic hepatitis B[J]. Diseases Research, 2024, 4( 1): 1- 2. DOI: 10.54457/DR.202401008. [26] NARMADA BC, KHAKPOOR A, SHIRGAONKAR N, et al. Single-cell landscape of functionally cured chronic hepatitis B patients reveals activation of innate and altered CD4-CTL-driven adaptive immunity[J]. J Hepatol, 2024, 81( 1): 42- 61. DOI: 10.1016/j.jhep.2024.02.017. [27] BERT NL, GILL US, HONG M, et al. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection[J]. Gastroenterology, 2020, 159( 2): 652- 664. DOI: 10.1053/j.gastro.2020.04.019. [28] TSENG TC, CHIANG C, LIU CJ, et al. Low hepatitis B core-related antigen levels correlate higher spontaneous seroclearance of hepatitis B surface antigen in chronic hepatitis B patients with high hepatitis B surface antigen levels[J]. Gastroenterology, 2023, 164( 4): 669- 679. e 6. DOI: 10.1053/j.gastro.2023.01.005. [29] WU JY, HE JY, XU HM. Global prevalence of occult HBV infection in children and adolescents: A systematic review and meta-analysis[J]. Ann Hepatol, 2024, 29( 1): 101158. DOI: 10.1016/j.aohep.2023.101158. [30] CHAN HLY, CHAN CK, HUI AJ, et al. Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA[J]. Gastroenterology, 2014, 146( 5): 1240- 1248. DOI: 10.1053/j.gastro.2014.01.044. [31] LU FM, WANG J, CHEN XM, et al. The potential use of serum HBV RNA to guide the functional cure of chronic hepatitis B[J]. Chin J Hepatol, 2017, 25( 2): 105- 110. DOI: 10.3760/cma.j.issn.1007-3418.2017.02.005.鲁凤民, 王杰, 陈香梅, 等. 乙型肝炎病毒RNA病毒样颗粒的发现及其对抗病毒治疗临床实践的潜在影响[J]. 中华肝脏病杂志, 2017, 25( 2): 105- 110. DOI: 10.3760/cma.j.issn.1007-3418.2017.02.005. -



 PDF下载 ( 1103 KB)
PDF下载 ( 1103 KB)

 下载:
下载:


