Expert recommendations on screening, testing and management for hepatitis B virus infection in adults
-
摘要: 乙型肝炎(乙肝)流行是重要的公共卫生问题,其疾病负担重。在我国,乙型肝炎的诊断率和治疗率与世界卫生组织(World Health Organization,WHO)提出的2030年消除病毒性肝炎公共卫生危害的目标仍有较大差距。为实现WHO和“2030健康中国”规划纲要目标,中华预防医学会组织国内临床、公共卫生和检验等领域专家,在全面回顾国内外相关文献的基础上,经过多轮讨论形成本建议,以实现对成人进行普遍筛查,对乙型肝炎病毒感染者进行评估、治疗和长期随访管理,对未感染者进行乙肝疫苗接种,消除乙型肝炎危害的目标。Abstract: The prevalence of hepatitis B represents a significant public health concern with a heavy disease burden. In China, there is still a big gap between the current diagnosis and treatment rates of hepatitis B and the goal of eliminating viral hepatitis as a public health threat by 2030 set by the World Health Organization (WHO). In order to achieve the WHO goal and the goal of 2030 Healthy China Outline, the Chinese Preventive Medicine Association organized domestic experts in the fields of clinical medicine, public health and clinical laboratory medicine to develop the
Expert Recommendations on Screening, Testing and Management for Hepatitis B Virus Infection in Adults after several rounds of discussion based on comprehensive review of relevant domestic and international guidelines and literatures, the purpose is to facilitate universal screening of hepatitis B virus (HBV) infection in adults and provide practical guidance on disease assessment, treatment and long-term follow-up management of people infected with HBV and vaccination for people susceptible to HBV infection, thus promoting the elimination of the threat of hepatitis B. -
Key words:
- Hepatitis B Virus /
- Screening /
- Testing /
- Management
-
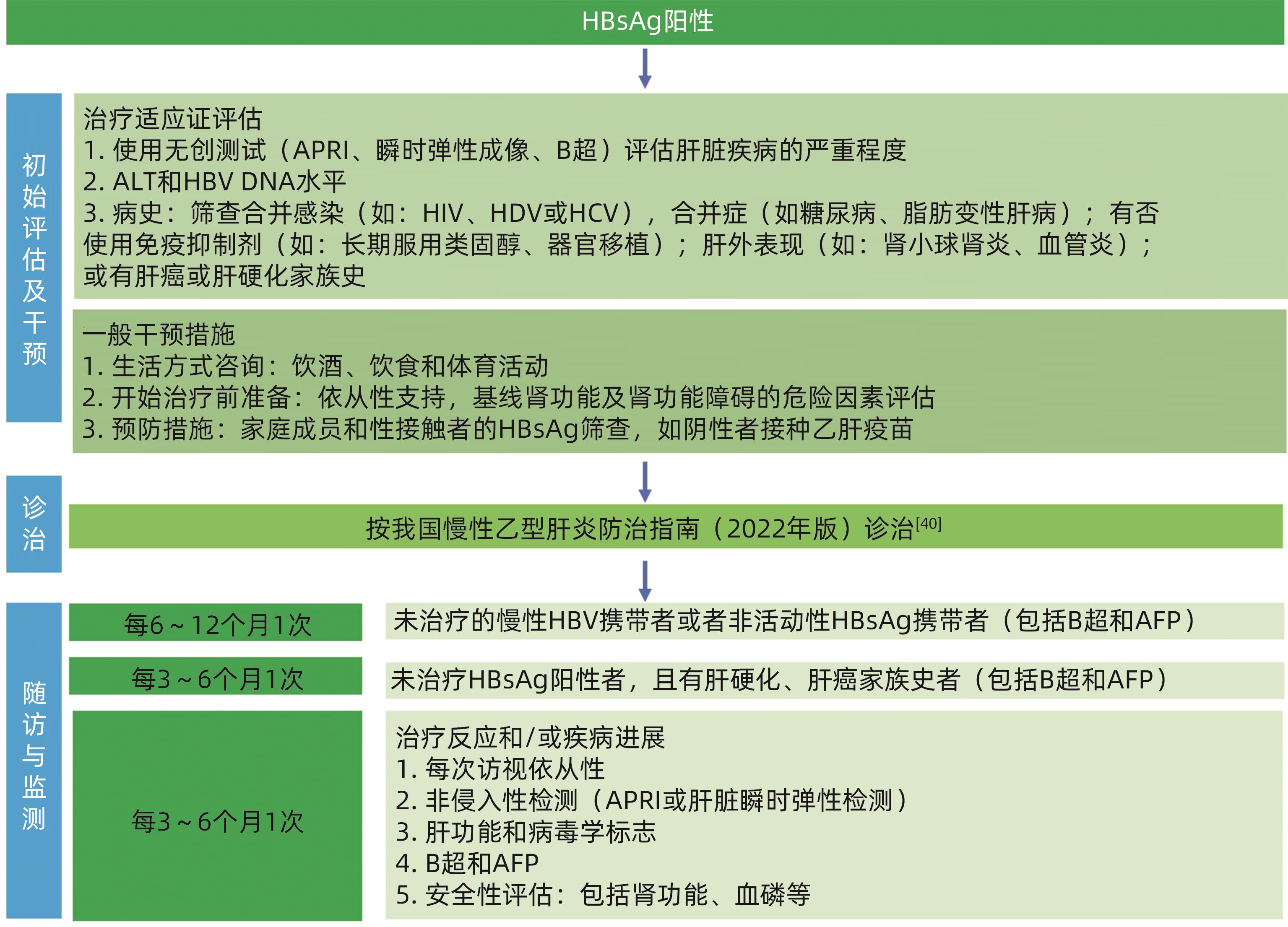
表 1 HBV 3项筛查结果解释和处理意见
Table 1. Result interpretation of three HBV screening items and treatment recommendations
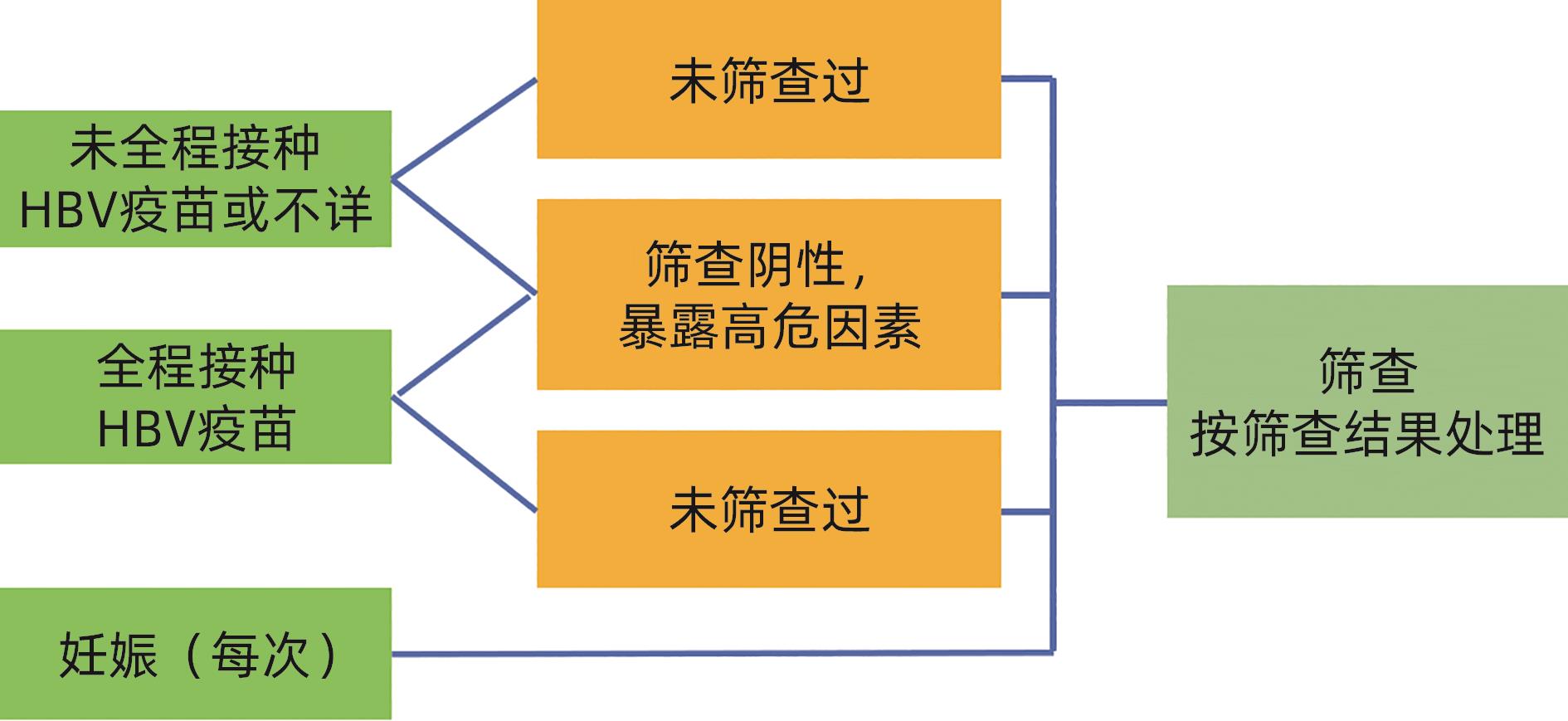
筛查结果 结果解释 处理意见 HBsAg 抗- HBs 抗- HBc - - - 易感,未感染过 建议接种乙肝疫苗 - + - 有免疫(接种过乙肝疫苗) 确认全程接种乙肝疫苗,如未全程,建议补种 + - + 急性或慢性感染 联系诊治 - + + 感染后康复 评价再活动风险 - - + 单项抗-HBc阳性 处理见表2 注:“-”表示筛查结果是阴性;“+”表示筛查结果是阳性。
表 2 单项抗-HBc阳性结果解释和处理意见
Table 2. Result interpretation of anti-HBc positivity and treatment recommendations
单项抗-HBc阳性结果解释 处理意见 既往感染,但抗-HBs消失 评价再活动风险 隐匿性乙肝 可抗病毒治疗 HBsAg突变株感染,所用试剂检测不到HBsAg 可抗病毒治疗 婴儿可由母亲被动输入抗-HBc 随访 抗-HBc假阳性 随访 -
[1] COOKE GS, FLOWER B, CUNNINGHAM E, et al. Progress towards elimination of viral hepatitis: A Lancet Gastroenterology& Hepatology Commission update[J]. Lancet Gastroenterol Hepatol, 2024, 9( 4): 346- 365. DOI: 10.1016/S2468-1253(23)00321-7. [2] TOY M, HUTTON D, JIA JD, et al. Costs and health impact of delayed implementation of a national hepatitis B treatment program in China[J]. J Glob Health, 2022, 12: 04043. DOI: 10.7189/jogh.12.04043. [3] PÉNEAU C, IMBEAUD S, BELLA TL, et al. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma[J]. Gut, 2022, 71( 3): 616- 626. DOI: 10.1136/gutjnl-2020-323153. [4] ERKEN R, LOUKACHOV V, van DORT K, et al. Quantified integrated hepatitis B virus is related to viral activity in patients with chronic hepatitis B[J]. Hepatology, 2022, 76( 1): 196- 206. DOI: 10.1002/hep.32352. [5] DONG Z, LI JR, ZHAO ZX, et al. Molecular epidemiology of hepatitis B virus genotypes and subgenotypes in ethnic minority populations, Yunnan Province, China[J]. Epidemiol Infect, 2021, 150: e11. DOI: 10.1017/S0950268821002326. [6] SORIANO V, ALVAREZ C, EDAGWA B, et al. Ultra-long-acting(XLA) antivirals for chronic viral hepatitis[J]. Int J Infect Dis, 2022, 114: 45- 50. DOI: 10.1016/j.ijid.2021.10.052. [7] KAKALOU C, POLYCHRONIDOU E, DROSOU V, et al. RiskRadar: Development and pilot results of a technical intervention targeting combination prevention regarding HIV, viral hepatitis, sexually transmitted infections and tuberculosis[J]. BMC Infect Dis, 2021, 21( Suppl 2): 866. DOI: 10.1186/s12879-021-06501-0. [8] CUI FQ, SHEN LP, LI L, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China[J]. Emerg Infect Dis, 2017, 23( 5): 765- 772. DOI: 10.3201/eid2305.161477. [9] LIU J, WANG XY, WANG Q, et al. Hepatitis B virus infection among 90 million pregnant women in 2853 Chinese Counties, 2015-2020: A national observational study[J]. Lancet Reg Health West Pac, 2021, 16: 100267. DOI: 10.1016/j.lanwpc.2021.100267. [10] HU M, CHEN W. Assessment of total economic burden of chronic hepatitis B(CHB)-related diseases in Beijing and Guangzhou, China[J]. Value Health, 2009, 12( Suppl 3): S89- S92. DOI: 10.1111/j.1524-4733.2009.00636.x. [11] PENG CY, CHIEN RN, LIAW YF. Hepatitis B virus-related decompensated liver cirrhosis: Benefits of antiviral therapy[J]. J Hepatol, 2012, 57( 2): 442- 450. DOI: 10.1016/j.jhep.2012.02.033. [12] FATTOVICH G, BORTOLOTTI F, DONATO F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors[J]. J Hepatol, 2008, 48( 2): 335- 352. DOI: 10.1016/j.jhep.2007.11.011. [13] ABARA WE, QASEEM A, SCHILLIE S, et al. Hepatitis B vaccination, screening, and linkage to care: Best practice advice from the American college of physicians and the centers for disease control and prevention[J]. Ann Intern Med, 2017, 167( 11): 794- 804. DOI: 10.7326/M17-1106. [14] TERRAULT NA, LOK ASF, MCMAHON BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance[J]. Hepatology, 2018, 67( 4): 1560- 1599. DOI: 10.1002/hep.29800. [15] TOY M, HUTTON D, HARRIS AM, et al. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States[J]. Clin Infect Dis, 2022, 74( 2): 210- 217. DOI: 10.1093/cid/ciab405. [16] XIAO YZ, HOWELL J, van GEMERT C, et al. Enhancing the hepatitis B care cascade in Australia: A cost-effectiveness model[J]. J Viral Hepat, 2020, 27( 5): 526- 536. DOI: 10.1111/jvh.13252. [17] WOLFFRAM I. A comprehensive screening for hepatitis B and C as an effective means of cancer prevention and as a prerequisite for elimination of chronic viral hepatitis-data and comments on a discussion[J]. Dtsch Med Wochenschr, 2023, 148( 4): 175- 182. DOI: 10.1055/a-1972-4118. [18] World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection[EB/OL].[ 2024-03-29]. https://www.who.int/publications/i/item/9789240090903. https://www.who.int/publications/i/item/9789240090903 [19] CONNERS EE, PANAGIOTAKOPOULOS L, HOFMEISTER MG, et al. Screening and testing for hepatitis B virus infection: CDC recommendations-United States, 2023[J]. MMWR Recomm Rep, 2023, 72( 1): 1- 25. DOI: 10.15585/mmwr.rr7201a1. [20] ALLARD NL, MACLACHLAN JH, TRAN L, et al. Time for universal hepatitis B screening for Australian adults[J]. Med J Aust, 2021, 215( 3): 103- 105. DOI: 10.5694/mja2.51114. [21] HARRIS AM, OSINUBI A, NELSON NP, et al. The hepatitis B care cascade using administrative claims data, 2016[J]. Am J Manag Care, 2020, 26( 8): 331- 338. DOI: 10.37765/ajmc.2020.44069. [22] OGAWA E, YEO YH, DANG N, et al. Diagnosis rates of chronic hepatitis B in privately insured patients in the United States[J]. JAMA Netw Open, 2020, 3( 4): e201844. DOI: 10.1001/jamanetworkopen.2020.1844. [23] VIJAYADEVA V, SPRADLING PR, MOORMAN AC, et al. Hepatitis B virus infection testing and prevalence among Asian and Pacific Islanders[J]. Am J Manag Care, 2014, 20( 4): e98- e104. [24] CDA Foundation. https://www.devex.com/organizations/center-for-disease-analysis-foundation-cdaf-132936. https://www.devex.com/organizations/center-for-disease-analysis-foundation-cdaf-132936 [25] CHEUNG KW, LAO TT. Hepatitis B-Vertical transmission and the prevention of mother-to-child transmission[J]. Best Pract Res Clin Obstet Gynaecol, 2020, 68: 78- 88. DOI: 10.1016/j.bpobgyn.2020.02.014. [26] JONAS MM. Hepatitis B and pregnancy: An underestimated issue[J]. Liver Int, 2009, 29( Suppl 1): 133- 139. DOI: 10.1111/j.1478-3231.2008.01933.x. [27] CUI F, WOODRING J, CHAN P, et al. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China[J]. Int J Epidemiol, 2018, 47( 5): 1529- 1537. DOI: 10.1093/ije/dyy077. [28] HAN GR, CAO MK, ZHAO W, et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection[J]. J Hepatol, 2011, 55( 6): 1215- 1221. DOI: 10.1016/j.jhep.2011.02.032. [29] MIN DY, HUANG WY, YANG JY, et al. Familial aggregation of hepatitis B virus infection and its influence factors in minority areas of Guizhou Province[J]. Chin J Public Health, 2016, 32( 2): 183- 187. DOI: 10.11847/zgggws2016-32-02-15.闵定玉, 黄文湧, 杨敬源, 等. 贵州少数民族人群HBV感染家庭聚集性及影响因素分析[J]. 中国公共卫生, 2016, 32( 2): 183- 187. DOI: 10.11847/zgggws2016-32-02-15. [30] YU YK, ZHU X, CHEN ZX, et al. Status and influencing factors of new hepatitis B virus infection in anzhou district, Mianyang city[J]. Acta Acad Med Sin, 2022, 44( 6): 996- 1003. DOI: 10.3881/j.issn.1000-503X.15058.余雨珂, 朱霞, 陈梓萱, 等. 绵阳市安州区居民乙型肝炎病毒新发感染情况及其影响因素[J]. 中国医学科学院学报, 2022, 44( 6): 996- 1003. DOI: 10.3881/j.issn.1000-503X.15058. [31] YUEN MF. Need to improve awareness and management of hepatitis B reactivation in patients receiving immunosuppressive therapy[J]. Hepatol Int, 2016, 10( 1): 102- 105. DOI: 10.1007/s12072-015-9694-1. [32] PÉREZ-ALVAREZ R, DÍAZ-LAGARES C, GARCÍA-HERNÁNDEZ F, et al. Hepatitis B virus(HBV) reactivation in patients receiving tumor necrosis factor(TNF)-targeted therapy: Analysis of 257 cases[J]. Medicine, 2011, 90( 6): 359- 371. DOI: 10.1097/MD.0b013e3182380a76. [33] YOO S, LEE DB, SHIM JH, et al. Risk of hepatitis B virus reactivation in patients treated with immunotherapy for anti-cancer treatment[J]. Clin Gastroenterol Hepatol, 2022, 20( 4): 898- 907. DOI: 10.1016/j.cgh.2021.06.019. [34] BAO YP, LARNEY S, PEACOCK A, et al. Prevalence of HIV, HCV and HBV infection and sociodemographic characteristics of people who inject drugs in China: A systematic review and meta-analysis[J]. Int J Drug Policy, 2019, 70: 87- 93. DOI: 10.1016/j.drugpo.2019.05.005. [35] ZHANG FJ, ZHU H, WU YS, et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010-12: A retrospective observational cohort study[J]. Lancet Infect Dis, 2014, 14( 11): 1065- 1072. DOI: 10.1016/S1473-3099(14)70946-6. [36] YANG J, RAO HY. Hepatitis B reactivation associated with hepatitis C antiviral therapy[J]. Chin Hepatol, 2018, 23( 7): 568- 570. DOI: 10.14000/j.cnki.issn.1008-1704.2018.07.002.杨甲, 饶慧瑛. 丙型肝炎抗病毒治疗相关的乙型肝炎再激活[J]. 肝脏, 2018, 23( 7): 568- 570. DOI: 10.14000/j.cnki.issn.1008-1704.2018.07.002. [37] XU YL, YANG XS, LIU KY, et al. Prevalence of and associated factors for HIV and HBV infections among men who have sex with men in Beijing, China[J]. J Cap Med Univ, 2014, 35( 1): 96- 100. DOI: 10.3969/j.issn.1006-7795.2014.01.021.许元龙, 杨仙珊, 刘凯燕, 等. 北京男男性接触人群性传播感染HIV/HBV现状及相关因素研究[J]. 首都医科大学学报, 2014, 35( 1): 96- 100. DOI: 10.3969/j.issn.1006-7795.2014.01.021. [38] ZHANG X, ZHU X, JI Y, et al. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus[J]. Biosci Rep, 2019, 39( 3): BSR20181715. DOI: 10.1042/BSR20181715. [39] CAMPBELL C, WANG T, MCNAUGHTON AL, et al. Risk factors for the development of hepatocellular carcinoma(HCC) in chronic hepatitis B virus(HBV) infection: A systematic review and meta-analysis[J]. J Viral Hepat, 2021, 28( 3): 493- 507. DOI: 10.1111/jvh.13452. [40] Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. J Pract Hepatol, 2023, 26( 3): S18- S39. DOI: 10.3969/j.issn.1672-5069.2023.03.040.中华医学会肝病学分会, 中华医学会感染病学分会. 慢性乙型肝炎防治指南(2022年版)[J]. 实用肝脏病杂志, 2023, 26( 3): S18- S39. DOI: 10.3969/j.issn.1672-5069.2023.03.040. [41] LI D, MALLORY T, SATOMURA S. AFP-L3: A new generation of tumor marker for hepatocellular carcinoma[J]. Clin Chim Acta, 2001, 313( 1-2): 15- 19. DOI: 10.1016/s0009-8981(01)00644-1. [42] NGUYEN HB, LE XT, NGUYEN HH, et al. Diagnostic value of hTERT mRNA and in combination with AFP, AFP-L3%, des-γ-carboxyprothrombin for screening of hepatocellular carcinoma in liver cirrhosis patients HBV or HCV-related[J]. Cancer Inform, 2022, 21: 11769351221100730. DOI: 10.1177/11769351221100730. [43] Society of Prevention and Control of Infectious Diseases, Chinese Preventive Medicine Association. Expert consensus on the role of hematological markers in the early clinical screening of hepatocellular carcinoma[J]. Chin J Viral Dis, 2021, 11( 5): 334- 340. DOI: 10.16505/j.2095-0136.2021.0049.中华预防医学会感染性疾病防控分会. 血液标志物用于临床肝细胞癌早期筛查的专家共识[J]. 中国病毒病杂志, 2021, 11( 5): 334- 340. DOI: 10.16505/j.2095-0136.2021.0049. [44] LIN XJ, CHONG YT, GUO ZW, et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study[J]. Lancet Oncol, 2015, 16( 7): 804- 815. DOI: 10.1016/S1470-2045(15)00048-0. [45] ZHU W, SHI P, LIANG A, et al. The combination of serum oligosaccharide chain(G-test), alpha-fetoprotein, and aspartate aminotransferase to alanine aminotransferase ratio provides the optimal diagnostic value for early detection of hepatocellular carcinoma[J]. BMC Cancer, 2022, 22( 1): 1061. DOI: 10.1186/s12885-022-10139-9. [46] Working Committee of Promoting the Elimination of Viral Hepatitis of Chinese Preventive Medicine Association, Society of Prevention and Control of Infectious Diseases of Chinese Preventive Medicine Association. Expert recommendations on hepatitis B vaccination in adults[J]. Chin J Viral Dis, 2024, 14( 4): 310- 316. DOI: 10.16505/j.2095-0136.2024.0047.中华预防医学会促进消除病毒性肝炎工作委员会, 中华预防医学会感染性疾病防控分会. 成人乙型肝炎疫苗接种专家建议[J]. 中国病毒病杂志, 2024, 14( 4): 310- 316. DOI: 10.16505/j.2095-0136.2024.0047. -



 PDF下载 ( 1971 KB)
PDF下载 ( 1971 KB)

 下载:
下载: