中草药相关肝损伤的表型特征及毒理机制
DOI: 10.12449/JCH240804
Phenotypic characteristics and toxicological mechanisms of herb-induced liver injury
-
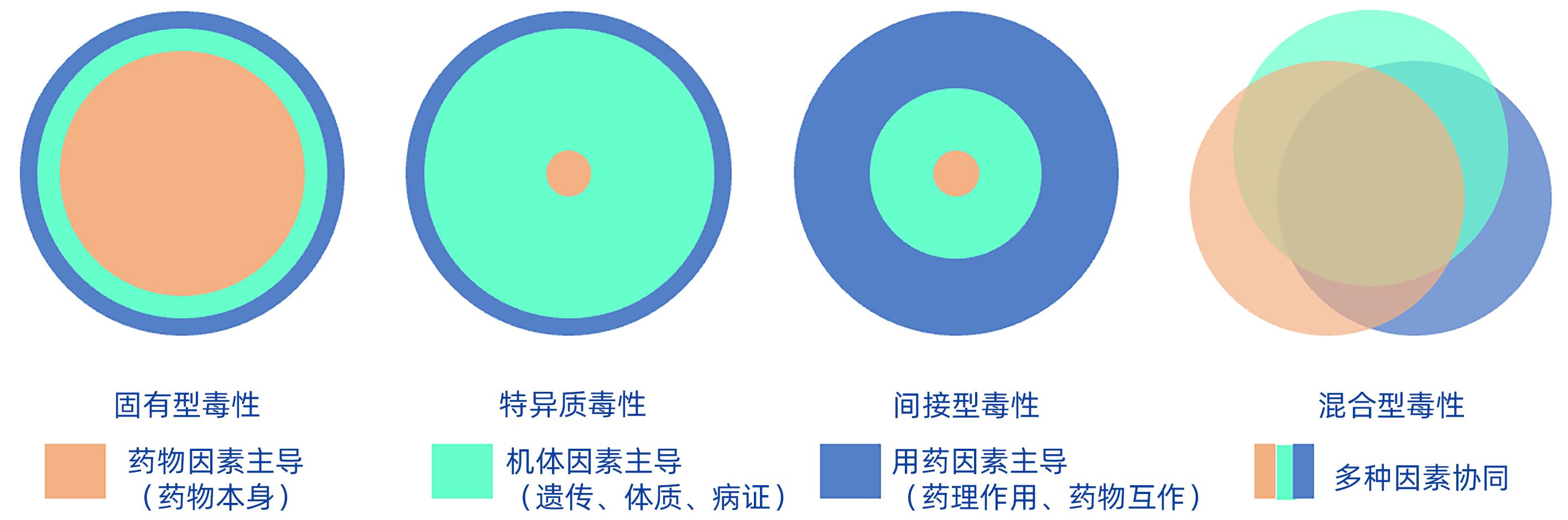
摘要: 为科学看待中草药安全性问题,进一步满足人们日益增长的健康需求,与时俱进深化中草药肝损伤研究尤为必要。本文从中草药相关肝损伤的表型特征及毒理机制两方面开展论述,强调不能局限于过去传统中草药固有毒性的认知模式,还应认识到特异质毒性、间接型毒性和混合型毒性等新的类型,这些类型为认识中草药相关肝损伤毒理机制提供了全新视角,对了解中草药相关肝损伤表型和毒理机制十分重要。
-
关键词:
- 中草药 /
- 化学性与药物性肝损伤 /
- 中药毒理学
Abstract: In order to deal with the problem of the safety of Chinese herbal medicine in a scientific way and further meet the growing health needs of people, it is particularly important to deepen the research on herb-induced liver injury. This article elaborates on the phenotypic characteristics and toxicological mechanisms of herb-induced liver injury and emphasizes that it should not only rely on the previous knowledge of the toxicity of Chinese herbal medicine, but also understand the new types of idiosyncratic toxicity, indirect toxicity, and mixed toxicity, which provide a new perspective for understanding the toxicological mechanisms of herb-induced liver injury and are of great importance to investigate the phenotype and toxicological mechanism of herb-induced liver injury. -
表 1 HILI的表型特征
Table 1. The phenotypic characteristics of HILI
分型方法 代表中药或影响因素 根据受损靶细胞分型 肝细胞损伤型 黄药子、雷公藤等 胆汁淤积型 苍耳子、白鲜皮、番泻叶等 混合型 决明子、细辛等 肝血管损伤型 土三七、千里光等 根据病程分型 急性 各种肝毒性中药及产品 慢性 何首乌、雷公藤、黄药子等 根据严重程度分型 轻度 与药物的剂量大小、服用时间的长短、机体差异、联合用药间相互作用等多种因素有关 中度 重度 肝衰竭 致死 根据组织病理学分型 炎症坏死型 闹羊花、附子等 胆汁淤积型 苍耳子、雷公藤、白鲜、黄药子、番泻叶等 脂肪肝和脂肪肝炎型 大枫子 血管损伤型 土三七、千里光等 表 2 中草药肝毒性类型的特点及其毒理机制
Table 2. Characteristics and toxicological mechanisms of hepatotoxicity with HILI
项目 固有型毒性 特异质毒性 间接型毒性 混合型毒性 主导因素 药物 遗传、体质、病证 药理作用、药物相互作用 药物、机体及用药协同 用药疗程 相关 无关 不确定 部分相关 药物剂量 相关 无关 不确定 部分相关 可预测 可预测 不可预测 不确定 部分可预测 潜伏期 短 长短不一 短 长短不一 肝损伤类型 肝细胞损伤型为主 肝细胞损伤型、 胆汁淤积型常见 肝细胞损伤型为主 肝细胞损伤型、 胆汁淤积型常见 代表中药 商陆、黄药子、川楝子、雷公藤、苍耳子、土三七等 何首乌等 淫羊藿等 补骨脂联合淫羊藿等 毒理机制 细胞色素P450酶表达异常、线粒体损伤、氧化应激损伤、炎症反应等 免疫炎症反应诱发 特异质肝损伤 药物代谢成分或药物间相互作用造成肝损伤 线粒体损伤、氧化应激 损伤、免疫炎症损伤等 -
[1] DU GH, LI L, YANG XY, et al. An overview of the ancient and modern researches on the toxicity of Chinese medicinal materials[J]. Pharmacol Clin Chin Mater Med, 2018, 34( 4): 187- 189. DOI: 10.13412/j.cnki.zyyl.2018.04.045.杜冠华, 李莉, 杨秀颖, 等. 中药毒之古今研究概况分析[J]. 中药药理与临床, 2018, 34( 4): 187- 189. DOI: 10.13412/j.cnki.zyyl.2018.04.045. [2] Branch of Hepatobiliary Diseases, China Association of Chinese Medicine; Branch of Chinese Patent Medicine, China Association of Chinese Medicine. Guideline for diagnosis and treatment of herb-induced liver injury[J]. J Clin Hepatol, 2016, 32( 5): 835- 843. DOI: 10.3969/j.issn.1001-5256.2016.05.003.中华中医药学会肝胆病分会, 中华中医药学会中成药分会. 中草药相关肝损伤临床诊疗指南[J]. 临床肝胆病杂志, 2016, 32( 5): 835- 843. DOI: 10.3969/j.issn.1001-5256.2016.05.003. [3] WANG JB, ZHU Y, BAI ZF, et al. Guidelines for the diagnosis and management of herb-induced liver injury[J]. Chin J Integr Med, 2018, 24( 9): 696- 706. DOI: 10.1007/s11655-018-3000-8. [4] HU ZX, ZHOU SZ, ZHANG N, et al. Development of polyclonal antibodies for detection of diosbulbin B-derived cis-enedial protein adducts[J]. Chem Res Toxicol, 2018, 31( 4): 231- 237. DOI: 10.1021/acs.chemrestox.7b00299. [5] LIU BS, WANG GJ, WANG ZH, et al. One case of acute liver injury caused by tripterygium glycosides tablets[J]. Chin J Pharmacovigil, 2023, 20( 5): 511- 513, 535. DOI: 10.19803/j.1672-8629.20220672.刘宝生, 王冠杰, 王振华, 等. 雷公藤多苷片致急性肝损伤1例分析[J]. 中国药物警戒, 2023, 20( 5): 511- 513, 535. DOI: 10.19803/j.1672-8629.20220672. [6] WANG MH, WANG X, YAN YJ, et al. Correlation between triptolide in Tripterygium glycoside tablets and hepatotoxicity in vitro[J]. Mod Chin Med, 2021, 23( 8): 1344- 1351. DOI: 10.13313/j.issn.1673-4890.20200520001.王曼虹, 王雪, 颜玉静, 等. 雷公藤多苷片中雷公藤甲素与体外肝毒性相关性研究[J]. 中国现代中药, 2021, 23( 8): 1344- 1351. DOI: 10.13313/j.issn.1673-4890.20200520001. [7] GE FL, NIU M, HAN ZX, et al. Analysis of epidemiological characteristics of drug induced liver injury associated with Baixianpi Preparations[J]. China J Chin Mater Med, 2019, 44( 5): 1048- 1052. DOI: 10.19540/j.cnki.cjcmm.20181217.001.葛斐林, 牛明, 韩紫欣, 等. 白鲜皮制剂相关肝损伤的药物流行病学特征分析[J]. 中国中药杂志, 2019, 44( 5): 1048- 1052. DOI: 10.19540/j.cnki.cjcmm.20181217.001. [8] ZHAO SQ, WU M. Acute renal failure and liver injury caused by Xanthium seed poisoning: a case report[J]. J Pract Diagn Ther, 2004, 18( 6): 514. DOI: 10.3969/j.issn.1674-3474.2004.06.036.赵胜乾, 吴敏. 苍耳子中毒致急性肾功能衰竭及肝损伤1例[J]. 实用诊断与治疗杂志, 2004, 18( 6): 514. DOI: 10.3969/j.issn.1674-3474.2004.06.036. [9] PENG WX, LI X, LIU FQ. Analysis of drug-induced liver injury induced by cassia seed extract: a case report[J]. Pharm J Chin People’s Liberation Army, 2016, 32( 5): 487. DOI: 10.3969/j.issn.1008-9926.2016.05.033.彭文绣, 李璇, 刘峰群. 决明子提取物致药物性肝损伤1例分析[J]. 解放军药学学报, 2016, 32( 5): 487. DOI: 10.3969/j.issn.1008-9926.2016.05.033. [10] ZHAO YM, WU L, ZHANG S, et al. Safety evaluation and risk control measures of Cassiae Semen[J]. China J Chin Mater Med, 2017, 42( 21): 4074- 4078. DOI: 10.19540/j.cnki.cjcmm.20170919.013.赵艺萌, 吴丽, 张烁, 等. 决明子的安全性评价与风险控制措施的探讨[J]. 中国中药杂志, 2017, 42( 21): 4074- 4078. DOI: 10.19540/j.cnki.cjcmm.20170919.013. [11] RAN S, FANG ZH, GONG Y, et al. Asarum brain tablets caused severe liver injury in 1 case[J]. Chin J Mod Appl Pharm, 2020, 37( 21): 2657- 2658. DOI: 10.13748/j.cnki.issn1007-7693.2020.21.017.冉姗, 方忠宏, 龚源, 等. 细辛脑片致严重肝损伤1例[J]. 中国现代应用药学, 2020, 37( 21): 2657- 2658. DOI: 10.13748/j.cnki.issn1007-7693.2020.21.017. [12] SUN MH, LIU YY, LEI X, et al. Hepatic veno-occlusive syndrome and multiple organ dysfunction caused by food-borne poisoning of Panax notoginseng: Report of one case[J/CD]. Chin J Hyg Rescue Electron Ed, 2019, 5( 3): 191- 192. DOI: 10.3877/cma.j.issn.2095-9133.2019.03.017.孙明辉, 刘园园, 雷旭, 等. 土三七中毒(食源性)致肝小静脉闭塞综合征并多器官功能损害1例[J/CD]. 中华卫生应急电子杂志, 2019, 5( 3): 191- 192. DOI: 10.3877/cma.j.issn.2095-9133.2019.03.017. [13] PAN HL, PAN WJ, CHEN CY, et al. Progress in the study of the effects of medicinal components of traditional Chinese medicine on liver toxicity[J]. Chin J Clin Ration Drug Use, 2020, 13( 27): 179- 181. DOI: 10.15887/j.cnki.13-1389/r.2020.27.078.潘海琳, 潘伟健, 陈楚裕, 等. 中药药效成分对肝脏毒性影响的研究进展[J]. 临床合理用药杂志, 2020, 13( 27): 179- 181. DOI: 10.15887/j.cnki.13-1389/r.2020.27.078. [14] Technology Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association; Study Group of Drug Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases. Chinese guideline for diagnosis and management of drug-induced liver injury(2023 version)[J]. Chin J Gastroenterol, 2023, 28( 7): 397- 431. DOI: 10.3760/cma.j.cn501113-20230419-00176.中国医药生物技术协会药物性肝损伤防治技术专业委员会, 中华医学会肝病学分会药物性肝病学组. 中国药物性肝损伤诊治指南(2023年版)[J]. 胃肠病学, 2023, 28( 7): 397- 431. DOI: 10.3760/cma.j.cn501113-20230419-00176. [15] LI M, LI RR, LIU CH. Clinic diagnosis and treatment of herb-induced liver injury[J]. Chin J Pharmacovigil, 2023, 20( 2): 127- 131. DOI: 10.19803/j.1672-8629.20220661.李盟, 李容容, 刘成海. 中草药引起肝损伤的临床诊治与用药[J]. 中国药物警戒, 2023, 20( 2): 127- 131. DOI: 10.19803/j.1672-8629.20220661. [16] WANG CY, DENG Y, LI P, et al. Prediction of biochemical nonresolution in patients with chronic drug-induced liver injury: A large multicenter study[J]. Hepatology, 2022, 75( 6): 1373- 1385. DOI: 10.1002/hep.32283. [17] HUANG A, ZHU Y, LIU SH, et al. An optimized short-term steroid therapy for chronic drug-induced liver injury: A prospective randomized clinical trial[J]. Liver Int, 2024, 44( 6): 1435- 1447. DOI: 10.1111/liv.15899. [18] CHEN H, ZHAO SS, YANG WJ. Clinical characteristics and outcome of patients with chronic drug-induced liver injury[J]. Chin Hepatol, 2022, 27( 6): 679- 682. DOI: 10.14000/j.cnki.issn.1008-1704.2022.06.013.陈红, 赵莎莎, 杨婉君. 慢性药物性肝损伤患者临床特征及转归分析[J]. 肝脏, 2022, 27( 6): 679- 682. DOI: 10.14000/j.cnki.issn.1008-1704.2022.06.013. [19] YANG YF. Pathological features and pathological diagnosis of drug-induced liver injury[J]. J Clin Hepatol, 2021, 37( 11): 2530- 2533. DOI: 10.3969/j.issn.1001-5256.2021.11.005.杨永峰. 药物性肝损伤病理学的特征及鉴别诊断[J]. 临床肝胆病杂志, 2021, 37( 11): 2530- 2533. DOI: 10.3969/j.issn.1001-5256.2021.11.005. [20] JIANG LN, ZHAO JM. Clinicopathological basis of liver failure[J]. J Clin Hepatol, 2019, 35( 9): 1916- 1919. DOI: 10.3969/j.issn.1001-5256.2019.09.005.蒋丽娜, 赵景民. 肝衰竭的临床病理基础[J]. 临床肝胆病杂志, 2019, 35( 9): 1916- 1919. DOI: 10.3969/j.issn.1001-5256.2019.09.005. [21] ZHAN XY, LUO Q, XIONG WY, et al. Comparison of effects of different aconite products on liver injury toxicity[J]. Lishizhen Med Mater Med Res, 2023, 34( 3): 609- 613. DOI: 10.3969/j.issn.1008-0805.2023.03.25.占心佾, 罗青, 熊文颖, 等. 不同附子炮制品对肝损伤毒性的作用比较[J]. 时珍国医国药, 2023, 34( 3): 609- 613. DOI: 10.3969/j.issn.1008-0805.2023.03.25. [22] GAO Y, XIE LY, LU ZQ. Research progress on toxicity of aconite and compatibility of attenuating toxicity and enhancing effect[J]. Tianjin Pharm, 2020, 32( 1): 65- 69. DOI: 10.3969/j.issn.1006-5687.2020.01.018.高艳, 谢灵燕, 卢志强. 附子毒理及减毒增效配伍的研究进展[J]. 天津药学, 2020, 32( 1): 65- 69. DOI: 10.3969/j.issn.1006-5687.2020.01.018. [23] WANG TL, ZHAO XY, SHAO C, et al. A proposed pathologic sub-classification of drug-induced liver injury[J]. Hepatol Int, 2019, 13( 3): 339- 351. DOI: 10.1007/s12072-019-09940-9. [24] SHI W, GAO Y, GUO YM, et al. Idiosyncratic hepatotoxicity evaluation of Cortex Dictamni based on immune stress[J]. Acta Pharm Sin, 2019, 54( 4): 678- 686. DOI: 10.16438/j.0513-4870.2018-1045.石伟, 高源, 郭玉明, 等. 基于免疫应激的白鲜皮致特异质肝损伤评价研究[J]. 药学学报, 2019, 54( 4): 678- 686. DOI: 10.16438/j.0513-4870.2018-1045. [25] CHEN H, GAO SQ, XIAO JR, et al. Clinical analysis of drug-induced liver damage caused by huangyaozi(Dioscorea bulbifera L.) and its preparation[J]. J Hunan Univ Chin Med, 2021, 41( 9): 1442- 1446. DOI: 10.3969/j.issn.1674-070X.2021.09.023.陈红, 高水群, 肖锦仁, 等. 黄药子及其制剂致药物性肝损伤病例临床分析[J]. 湖南中医药大学学报, 2021, 41( 9): 1442- 1446. DOI: 10.3969/j.issn.1674-070X.2021.09.023. [26] QIU BY, ZHANG BY, HUANG NL. A commonly used Chinese medicine that can cause liver side effects[J]. Asia Pac Tradit Med, 2011, 7( 8): 188- 189.邱宝玉, 张碧玉, 黄南龙. 可引起肝脏不良反应的常用中药[J]. 亚太传统医药, 2011, 7( 8): 188- 189. [27] HE TT, GONG M, WANG LF, et al. Prospective study on pathological features and traditional Chinese medicine syndrome types of patients with herb-induced liver injury[J]. Chin J Integr Tradit West Med, 2019, 39( 8): 932- 936. DOI: 10.7661/j.cjim.20190318.044.何婷婷, 宫嫚, 王立福, 等. 中药肝损伤病例的病理特征及中医证型前瞻性研究[J]. 中国中西医结合杂志, 2019, 39( 8): 932- 936. DOI: 10.7661/j.cjim.20190318.044. [28] HOOFNAGLE JH, BJÖRNSSON ES. Drug-induced liver injury-types and phenotypes[J]. N Engl J Med, 2019, 381( 3): 264- 273. DOI: 10.1056/NEJMra1816149. [29] GAO YJ, ZHAO X, BAI ZF, et al. Prevention and control of safety risks of traditional Chinese medicine based on indirect knowledge of toxicity[J]. Chin J Pharmacovigil, 2021, 18( 11): 1004- 1008. DOI: 10.19803/j.1672-8629.2021.11.02.高云娟, 赵旭, 柏兆方, 等. 基于间接毒性认知的中药安全风险防控[J]. 中国药物警戒, 2021, 18( 11): 1004- 1008. DOI: 10.19803/j.1672-8629.2021.11.02. [30] XIAO XH, ZHAO X, BAI ZF, et al. New outlook on safety of traditional Chinese medicine: Concept and practice[J]. China J Chin Mater Med, 2023, 48( 10): 2557- 2564. DOI: 10.19540/j.cnki.cjcmm.20230309.601.肖小河, 赵旭, 柏兆方, 等. 中药新安全观及实践[J]. 中国中药杂志, 2023, 48( 10): 2557- 2564. DOI: 10.19540/j.cnki.cjcmm.20230309.601. [31] BAI ZF, WANG JB, XIAO XH. Cognition innovation of toxicity of Chinese medicine and safe and precise medication[J]. China J Chin Mater Med, 2022, 47( 10): 2557- 2564. DOI: 10.19540/j.cnki.cjcmm.20220211.601.柏兆方, 王伽伯, 肖小河. 中药毒性认知创新与安全精准用药[J]. 中国中药杂志, 2022, 47( 10): 2557- 2564. DOI: 10.19540/j.cnki.cjcmm.20220211.601. [32] YU LQ, ZHENG J, LI JY, et al. Mechanism of hepatotoxicity induced by neem seed in mice based on serum exosome and liver miRNA expression profiles[C]// The Professional Committee of Clinical Pharmacology and Toxicology of the Chinese Society of Integrated Traditional Chinese and Western Medicine. 2019 Abstracts of the Third Academic Seminar of the Professional Committee of Clinical Pharmacology and Toxicology of the Chinese Society of Integrated Traditional Chinese and Western Medicine. 2019: 2.俞铃琪, 郑洁, 李俊颖, 等. 基于血清外泌体和肝脏miRNA表达谱的川楝子致小鼠肝毒性机制研究[C]//中国中西医结合学会临床药理与毒理专业委员会. 2019中国中西医结合学会临床药理与毒理专业委员会第三届学术研讨会论文摘要集. 2019: 2. [33] JI C, ZHENG J, TONG W, et al. Revealing the mechanism of Fructus meliae toosendan-induced liver injury in mice by integrating microRNA and mRNA-based toxicogenomics data[J]. RSC Adv, 2015, 5( 100): 81774- 81783. DOI: 10.1039/C5RA10112C. [34] LIU Y, WU H, WANG Z, et al. Integrated expression profiles of mRNA and miRNA in a gerbil model of fatty liver fibrosis treated with exenatide[J]. Clin Res Hepatol Gastroenterol, 2021, 45( 2): 101312. DOI: 10.1016/j.clinre.2019.07.013. [35] YANG XW, ZHANG YH, LIU Y, et al. Emodin induces liver injury by inhibiting the key enzymes of FADH/NADPH transport in rat liver[J]. Toxicol Res, 2018, 7( 5): 888- 896. DOI: 10.1039/c7tx00307b. [36] HASNAT M, YUAN ZQ, NAVEED M, et al. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: A novel mechanism in triptolide-induced hepatotoxicity[J]. Cell Biol Toxicol, 2019, 35( 3): 267- 280. DOI: 10.1007/s10565-018-9447-8. [37] LI FJ, YAO GT, JIN RM, et al. Mechanism studies on hepatotoxicity of rats induced by Sophorae Tonkinensis radix et Rhizoma in rat[J]. China J Chin Mater Med, 2011, 36( 13): 1821- 1823. DOI: 10.4268/cjcmm20111327.李峰杰, 姚广涛, 金若敏, 等. 山豆根致大鼠肝毒性机制研究[J]. 中国中药杂志, 2011, 36( 13): 1821- 1823. DOI: 10.4268/cjcmm20111327. [38] LI CP, RAO T, CHEN XP, et al. HLA-B*35:01 allele is a potential biomarker for predicting Polygonum multiflorum-induced liver injury in humans[J]. Hepatology, 2019, 70( 1): 346- 357. DOI: 10.1002/hep.30660. [39] TU C. The identification for susceptible individuals of idiosyncratic liver injury induced by traditional Chinese medicines and rational use[D]. Chengdu: Chengdu University of TCM, 2019.涂灿. 中药特异质肝损伤易感人群识别及安全合理用药研究: 以何首乌为例[D]. 成都: 成都中医药大学, 2019. [40] ZHANG L, NIU M, WEI AW, et al. Risk profiling using metabolomic characteristics for susceptible individuals of drug-induced liver injury caused by Polygonum multiflorum[J]. Arch Toxicol, 2020, 94( 1): 245- 256. DOI: 10.1007/s00204-019-02595-3. [41] ZHANG L, BAI ZF, LI CY, et al. Study on idiosyncratic liver injury and content of cis-2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside in radix Polygoni multiflori Preparata[J]. Acta Pharm Sin, 2017, 52( 7): 1041- 1047. DOI: 10.16438/j.0513-4870.2017-0307.张乐, 柏兆方, 李春雨, 等. 制首乌中顺式二苯乙烯苷转化量与特异质肝损伤的相关性研究[J]. 药学学报, 2017, 52( 7): 1041- 1047. DOI: 10.16438/j.0513-4870.2017-0307. [42] ZHANG L, LIU XY, TU C, et al. Components synergy between stilbenes and emodin derivatives contributes to hepatotoxicity induced by Polygonum multiflorum[J]. Xenobiotica, 2020, 50( 5): 515- 525. DOI: 10.1080/00498254.2019.1658138. [43] GUAN SB, ZHANG WT, GUO H. A case of the syndrome of disappearing intrahepatic bile ducts caused by Polygonum multiflorum[J]. Chin J Hepatol, 2024, 32( 3): 248- 250. DOI: 10.3760/cma.j.cn501113-20231203-00260.关善斌, 张文涛, 郭卉. 何首乌致肝内胆管消失综合征1例[J]. 中华肝脏病杂志, 2024, 32( 3): 248- 250. DOI: 10.3760/cma.j.cn501113-20231203-00260. [44] WANG ZL. Susceptible components and mechanism studies of epimedii folium-induced immunological idiosyncratic liver injury based on NLRP3 inflammasome[D]. Chengdu: Chengdu University of TCM, 2021.王智磊. 基于NLRP3炎症小体的淫羊藿致免疫特异质肝损伤易感成分及作用机制研究[D]. 成都: 成都中医药大学, 2021. [45] LIU Q, GUO YL, DONG TW, et al. Research progress on hepatotoxicity mechanism and attenuation methods of psoraleae fructus[J]. Chin J Exp Tradit Med Formulae, 2021, 27( 11): 233- 239. DOI: 10.13422/j.cnki.syfjx.20210626.刘巧, 郭延丽, 董泰玮, 等. 补骨脂肝损伤机制及减毒方法研究进展[J]. 中国实验方剂学杂志, 2021, 27( 11): 233- 239. DOI: 10.13422/j.cnki.syfjx.20210626. [46] HUANG Y, LIU YL, MA RR, et al. Clinical case analysis and disassembled prescription study of liver injury related to Xianling Gubao[J]. Acta Pharm Sin, 2021, 56( 1): 266- 273. DOI: 10.16438/j.0513-4870.2020-1477.黄迎, 刘亚蕾, 马润然, 等. 仙灵骨葆相关肝损伤的临床病例分析及拆方实验研究[J]. 药学学报, 2021, 56( 1): 266- 273. DOI: 10.16438/j.0513-4870.2020-1477. [47] BAI ZF, MENG YK, HE LZ, et al. Immune idiosyncratic liver injury induced by traditional non-toxic traditional Chinese medicine and a hypothesis of its mechanism[J]. Chin Pharm J, 2017, 52( 13): 1105- 1109. DOI: 10.11669/cpj.2017.13.001.柏兆方, 孟雅坤, 贺兰芝, 等. 传统无毒中药诱导的免疫特异质型肝损伤及其机制假说[J]. 中国药学杂志, 2017, 52( 13): 1105- 1109. DOI: 10.11669/cpj.2017.13.001. [48] BAI ZF, GAO Y, ZUO XB, et al. Progress in research on the pathogenesis of immune regulation and idiosyncratic drug-induced liver injury[J]. Acta Pharm Sin, 2017, 52( 7): 1019- 1026. DOI: 10.16438/j.0513-4870.2017-0315.柏兆方, 高源, 左晓彬, 等. 免疫调控与特异质型药物性肝损伤发生机制研究进展[J]. 药学学报, 2017, 52( 7): 1019- 1026. DOI: 10.16438/j.0513-4870.2017-0315. [49] WANG ZL, XU G, WANG HB, et al. Icariside II, a main compound in Epimedii Folium, induces idiosyncratic hepatotoxicity by enhancing NLRP3 inflammasome activation[J]. Acta Pharm Sin B, 2020, 10( 9): 1619- 1633. DOI: 10.1016/j.apsb.2020.03.006. [50] GAO Y, XU G, MA L, et al. Icariside I specifically facilitates ATP or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic hepatotoxicity[J]. Cell Commun Signal, 2021, 19( 1): 13. DOI: 10.1186/s12964-020-00647-1. [51] FU SB, XU G, GAO Y, et al. Inhibitory effect and mechanism of licochalcone A on NLRP3 inflammasome[J]. Acta Pharm Sin, 2018, 53( 12): 2050- 2056. DOI: 10.16438/j.0513-4870.2018-0713.付书彬, 徐广, 高源, 等. 甘草查尔酮A对NLRP3炎症小体的调控作用及机制初探[J]. 药学学报, 2018, 53( 12): 2050- 2056. DOI: 10.16438/j.0513-4870.2018-0713. [52] WANG JB, CUI HR, BAI ZF, et al. Precision medicine-oriented safety assessment strategy for traditional Chinese medicines: Disease-syndrome-based toxicology[J]. Acta Pharm Sin, 2016, 51( 11): 1681- 1688. DOI: 10.16438/j.0513-4870.2016-0812.王伽伯, 崔鹤蓉, 柏兆方, 等. 精准医学下的中药安全性评价策略和方法: 病证毒理学[J]. 药学学报, 2016, 51( 11): 1681- 1688. DOI: 10.16438/j.0513-4870.2016-0812. -



 PDF下载 ( 955 KB)
PDF下载 ( 955 KB)

 下载:
下载:


