肝内胆管癌的诊疗进展
DOI: 10.12449/JCH240728
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:胡笛负责收集与分析资料,撰写论文;朱晓黎参与课题设计及指导;黄金涛、仲斌演、沈健参与论文修改;朱晓黎负责拟定写作思路,指导撰写文章并最后定稿。
-
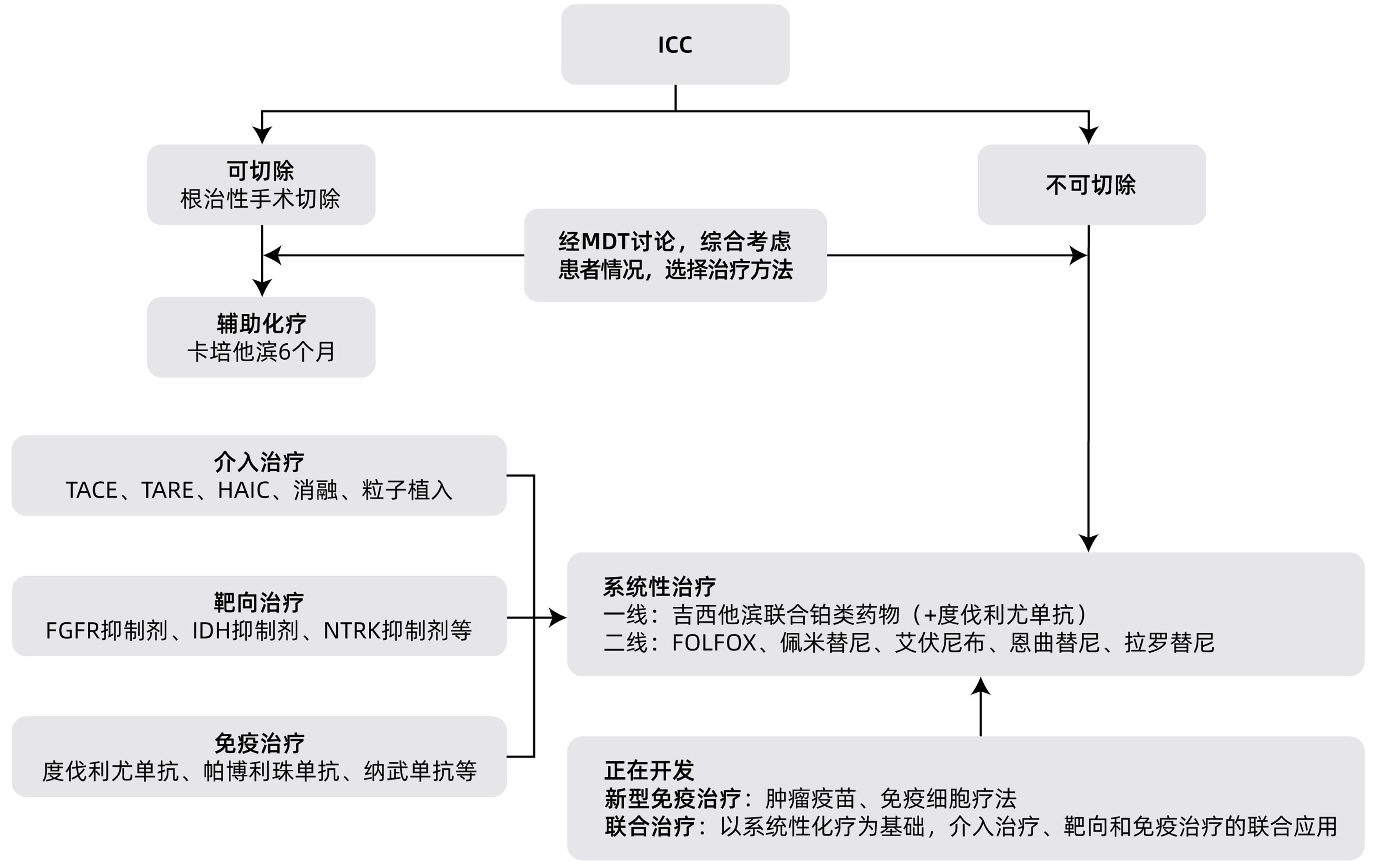
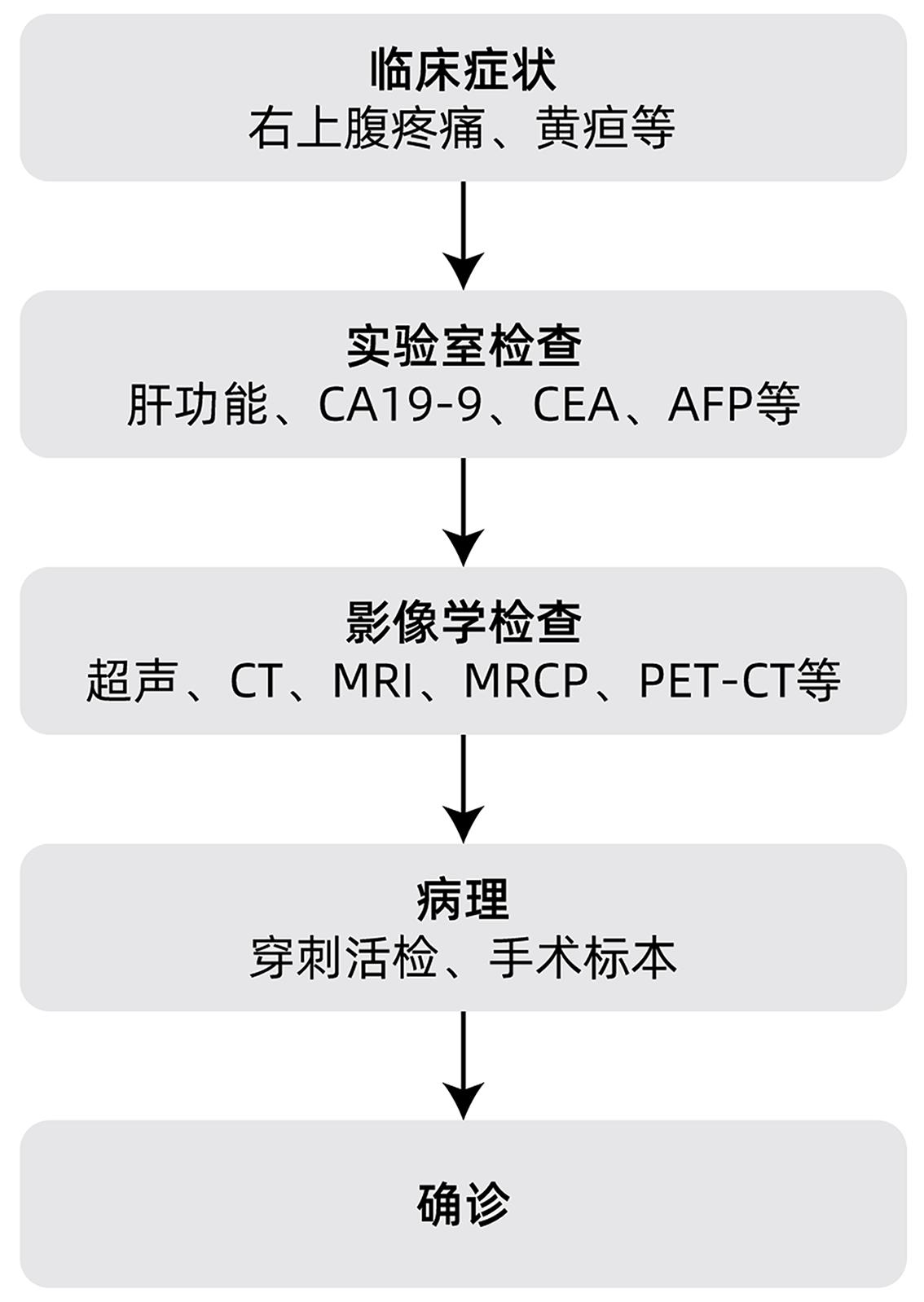
摘要: 肝内胆管癌(ICC)是原发性肝癌相对少见的类型,近年来发病率逐渐增加。由于其发病隐匿,临床症状不典型,大部分患者确诊时已处于疾病中晚期阶段,因此及时诊断和治疗非常重要。早期ICC患者的标准治疗方案是根治性手术切除,中晚期患者则以系统性化疗为基础,联合介入治疗、靶向和免疫治疗等。本文拟对ICC的诊疗进展进行综述。Abstract: Intrahepatic cholangiocarcinoma (ICC) is a relatively rare type of primary liver cancer, and its incidence rate has gradually increased in recent years. Due to its insidious onset and atypical clinical symptoms, most patients are already in the advanced stage of the disease at the time of confirmed diagnosis, and therefore, timely diagnosis and treatment are of great importance. Radical surgical resection is the standard treatment regimen for early-stage ICC patients, while systemic chemotherapy is the basic treatment for patients with advanced ICC and is often combined with interventional treatment, targeted therapy, and immunotherapy. This article reviews the advances in the diagnosis and treatment of ICC.
-
Key words:
- Cholangiocarcinoma /
- Diagnosis /
- Therapeutics
-
[1] SIEGEL RL, MILLER KD, FUCHS HE, et al. Cancer statistics, 2022[J]. CA Cancer J Clin, 2022, 72( 1): 7- 33. DOI: 10.3322/caac.21708. [2] DASGUPTA P, HENSHAW C, YOULDEN DR, et al. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis[J]. Front Oncol, 2020, 10: 171. DOI: 10.3389/fonc.2020.00171. [3] BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. [4] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [5] CHEN CB, NELSON LJ, ÁVILA MA, et al. Mitogen-activated protein kinases(MAPKs) and cholangiocarcinoma: The missing link[J]. Cells, 2019, 8( 10): 1172. DOI: 10.3390/cells8101172. [6] LEE AJ, CHUN YS. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates[J]. Chin Clin Oncol, 2018, 7( 5): 52. DOI: 10.21037/cco.2018.07.03. [7] MALAGUARNERA G, PALADINA I, GIORDANO M, et al. Serum markers of intrahepatic cholangiocarcinoma[J]. Dis Markers, 2013, 34( 4): 219- 228. DOI: 10.3233/DMA-130964. [8] MORO A, MEHTA R, SAHARA K, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol, 2020, 27( 8): 2888- 2901. DOI: 10.1245/s10434-020-08350-8. [9] OHAEGBULAM KC, KOETHE Y, FUNG A, et al. The multidisciplinary management of cholangiocarcinoma[J]. Cancer, 2023, 129( 2): 184- 214. DOI: 10.1002/cncr.34541. [10] LAPITZ A, AZKARGORTA M, MILKIEWICZ P, et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma[J]. J Hepatol, 2023, 79( 1): 93- 108. DOI: 10.1016/j.jhep.2023.02.027. [11] KIM DW, KIM SY, YOO C, et al. Update on biliary cancer imaging[J]. Radiol Clin North Am, 2022, 60( 5): 825- 842. DOI: 10.1016/j.rcl.2022.05.001. [12] FÁBREGA-FOSTER K, GHASABEH MA, PAWLIK TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma[J]. Hepatobiliary Surg Nutr, 2017, 6( 2): 67- 78. DOI: 10.21037/hbsn.2016.12.10. [13] Expert Committee of Expert Consensus on Pathological Diagnosis of Intrahepatic Cholangiocarcinoma( 2022 version). Expert consensus on pathological diagnosis of intrahepatic cholangiocarcinoma(2022 version)[J]. Chin J Pathol, 2022, 51( 9): 819- 827. DOI: 10.3760/cma.j.cn112151-20220517-00423.《肝内胆管癌病理诊断专家共识(2022版)》编写专家委员会. 肝内胆管癌病理诊断专家共识(2022版)[J]. 中华病理学杂志, 2022, 51( 9): 819- 827. DOI: 10.3760/cma.j.cn112151-20220517-00423. [14] SIA D, HOSHIDA Y, VILLANUEVA A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes[J]. Gastroenterology, 2013, 144( 4): 829- 840. DOI: 10.1053/j.gastro.2013.01.001. [15] KOMUTA M. Intrahepatic cholangiocarcinoma: Tumour heterogeneity and its clinical relevance[J]. Clin Mol Hepatol, 2022, 28( 3): 396- 407. DOI: 10.3350/cmh.2021.0287. [16] DONG LQ, LU DY, CHEN R, et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma[J]. Cancer Cell, 2022, 40( 1): 70- 87. DOI: 10.1016/j.ccell.2021.12.006. [17] CHUN YS, JAVLE M. Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma[J]. Cancer Control, 2017, 24( 3): 1073274817729241. DOI: 10.1177/1073274817729241. [18] NIU YJ, ZHA Y, LI SJ, et al. Analysis on prognosis related factors of patients with cholangiocarcinoma after radical resection and establishment of survival prediction model[J]. J Jilin Univ(Med Edit), 2022, 48( 4): 979- 987. DOI: 10.13481/j.1671-587X.20220418.牛英杰, 查勇, 李思嘉, 等. 胆管癌根治性切除术后患者预后相关因素分析和生存预测模型构建[J]. 吉林大学学报(医学版), 2022, 48( 4): 979- 987. DOI: 10.13481/j.1671-587X.20220418. [19] PRIMROSE JN, FOX RP, PALMER DH, et al. Capecitabine compared with observation in resected biliary tract cancer(BILCAP): A randomised, controlled, multicentre, phase 3 study[J]. Lancet Oncol, 2019, 20( 5): 663- 673. DOI: 10.1016/S1470-2045(18)30915-X. [20] Chinese Society of Liver Cancer Cholangiocarcinoma Cooperative Group. Chinese expert consensus on management of intrahepatic cholangiocarcinoma(2022 edition)[J]. Chin J Dig Surg, 2022, 21( 10): 1269- 1301. DOI: 10.3760/cma.j.cn115610-20220829-00476.中国抗癌协会肝癌专业委员会胆管癌协作组. 原发性肝癌诊疗指南之肝内胆管癌诊疗中国专家共识(2022版)[J]. 中华消化外科杂志, 2022, 21( 10): 1269- 1301. DOI: 10.3760/cma.j.cn115610-20220829-00476. [21] BOWLUS CL, ARRIVÉ L, BERGQUIST A, et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma[J]. Hepatology, 2023, 77( 2): 659- 702. DOI: 10.1002/hep.32771. [22] European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma[J]. J Hepatol, 2023, 79( 1): 181- 208. DOI: 10.1016/j.jhep.2023.03.010. [23] BUETTNER S, van VUGT JL, IJZERMANS JN, et al. Intrahepatic cholangiocarcinoma: Current perspectives[J]. Onco Targets Ther, 2017, 10: 1131- 1142. DOI: 10.2147/OTT.S93629. [24] BUETTNER S, KOERKAMP BG, EJAZ A, et al. The effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients-a multi-institutional analysis[J]. J Surg Oncol, 2017, 115( 3): 312- 318. DOI: 10.1002/jso.24524. [25] FANG TL, XIAO JY, ZHANG YR, et al. Combined with interventional therapy, immunotherapy can create a new outlook for tumor treatment[J]. Quant Imaging Med Surg, 2021, 11( 6): 2837- 2860. DOI: 10.21037/qims-20-173. [26] MARTIN RCG 2, SIMO KA, HANSEN P, et al. Drug-eluting bead, irinotecan therapy of unresectable intrahepatic cholangiocarcinoma(DELTIC) with concomitant systemic gemcitabine and cisplatin[J]. Ann Surg Oncol, 2022, 29( 9): 5462- 5473. DOI: 10.1245/s10434-022-11932-3. [27] EDELINE J, TOUCHEFEU Y, GUIU B, et al. Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. JAMA Oncol, 2020, 6( 1): 51- 59. DOI: 10.1001/jamaoncol.2019.3702. [28] CERCEK A, BOERNER T, TAN BR, et al. Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. JAMA Oncol, 2020, 6( 1): 60- 67. DOI: 10.1001/jamaoncol.2019.3718. [29] MEI J, TANG YH, WEI W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma[J]. Front Oncol, 2021, 11: 618206. DOI: 10.3389/fonc.2021.618206. [30] ZHANG QY, LIU XY, WEI SM, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: A single-arm, open-label, phase II study[J]. Front Oncol, 2021, 11: 751391. DOI: 10.3389/fonc.2021.751391. [31] VALLE J, WASAN H, PALMER DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. N Engl J Med, 2010, 362( 14): 1273- 1281. DOI: 10.1056/NEJMoa0908721. [32] MORIZANE C, OKUSAKA T, MIZUSAWA J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT(JCOG1113) randomized phase III clinical trial[J]. Ann Oncol, 2019, 30( 12): 1950- 1958. DOI: 10.1093/annonc/mdz402. [33] KIM ST, KANG JH, LEE J, et al. Capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers: A multicenter, open-label, randomized, phase III, noninferiority trial[J]. Ann Oncol, 2019, 30( 5): 788- 795. DOI: 10.1093/annonc/mdz058. [34] SHROFF RT, JAVLE MM, XIAO LC, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial[J]. JAMA Oncol, 2019, 5( 6): 824- 830. DOI: 10.1001/jamaoncol.2019.0270. [35] IOKA T, KANAI M, KOBAYASHI S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer(KHBO1401- MITSUBA)[J]. J Hepatobiliary Pancreat Sci, 2023, 30( 1): 102- 110. DOI: 10.1002/jhbp.1219. [36] LAMARCA A, PALMER DH, WASAN HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer(ABC-06): A phase 3, open-label, randomised, controlled trial[J]. Lancet Oncol, 2021, 22( 5): 690- 701. DOI: 10.1016/S1470-2045(21)00027-9. [37] VOGEL A, SEGATTO O, STENZINGER A, et al. FGFR2 inhibition in cholangiocarcinoma[J]. Annu Rev Med, 2023, 74: 293- 306. DOI: 10.1146/annurev-med-042921-024707. [38] ABOU-ALFA G, SAHAI V, HOLLEBECQUE A, et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma(CCA): Update of FIGHT-202[J]. J Clin Oncol, 2021, 39: 4086. DOI: 10.1200/JCO.2021.39.15_SUPPL.4086. [39] SHI GM, HUANG XY, WEN TF, et al. Pemigatinib in Chinese patients with advanced/metastatic or surgically unresectable cholangiocarcinoma including FGFR2 fusion or rearrangement: Updated data from an open-label, single-arm, multicenter phase II study(CIBI375A201 study)[J]. J Clin Oncol, 2022, 40( 16_suppl): e16183. DOI: 10.1200/jco.2022.40.16_suppl.e16183. [40] RIZZO A, RICCI AD, BRANDI G. IDH inhibitors in advanced cholangiocarcinoma: Another arrow in the quiver?[J]. Cancer Treat Res Commun, 2021, 27: 100356. DOI: 10.1016/j.ctarc.2021.100356. [41] ABOU-ALFA GK, MACARULLA T, JAVLE MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma(ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study[J]. Lancet Oncol, 2020, 21( 6): 796- 807. DOI: 10.1016/S1470-2045(20)30157-1. [42] DU JJ, LV X, ZHANG ZY, et al. Revisiting targeted therapy and immunotherapy for advanced cholangiocarcinoma[J]. Front Immunol, 2023, 14: 1142690. DOI: 10.3389/fimmu.2023.1142690. [43] DOEBELE RC, DRILON A, PAZ-ARES L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials[J]. Lancet Oncol, 2020, 21( 2): 271- 282. DOI: 10.1016/S1470-2045(19)30691-6. [44] DRILON A, LAETSCH TW, KUMMAR S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children[J]. N Engl J Med, 2018, 378( 8): 731- 739. DOI: 10.1056/NEJMoa1714448. [45] VALLE JW, LAMARCA A, GOYAL L, et al. New horizons for precision medicine in biliary tract cancers[J]. Cancer Discov, 2017, 7( 9): 943- 962. DOI: 10.1158/2159-8290.CD-17-0245. [46] OHBA A, MORIZANE C, UENO M, et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial[J]. Future Oncol, 2022, 18( 19): 2351- 2360. DOI: 10.2217/fon-2022-0214. [47] ZUO S, CHEN Q, ZOU WL. Current status and prospect of immunotherapy for cholangiocarcinoma[J]. Chin J Dig Surg, 2022, 21( 7): 873- 879. DOI: 10.3760/cma.j.cn115610-20220506-00254.左石, 陈乾, 邹卫龙. 胆管癌免疫治疗的现状与展望[J]. 中华消化外科杂志, 2022, 21( 7): 873- 879. DOI: 10.3760/cma.j.cn115610-20220506-00254. [48] BERI N. Immune checkpoint inhibitors in cholangiocarcinoma[J]. Immunotherapy, 2023, 15( 7): 541- 551. DOI: 10.2217/imt-2022-0288. [49] MARABELLE A, LE DT, ASCIERTO PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study[J]. J Clin Oncol, 2020, 38( 1): 1- 10. DOI: 10.1200/JCO.19.02105. [50] BENSON AB, D’ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19( 5): 541- 565. DOI: 10.6004/jnccn.2021.0022. [51] OVERMAN MJ, LONARDI S, WONG KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer[J]. J Clin Oncol, 2018, 36( 8): 773- 779. DOI: 10.1200/JCO.2017.76.9901. [52] HODI FS, CHIARION-SILENI V, GONZALEZ R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma(CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial[J]. Lancet Oncol, 2018, 19( 11): 1480- 1492. DOI: 10.1016/S1470-2045(18)30700-9. [53] IOKA T, UENO M, OH DY, et al. Evaluation of safety and tolerability of durvalumab(D) with or without tremelimumab(T) in patients(pts) with biliary tract cancer(BTC)[J]. J Clin Oncol, 2019, 37( 4_suppl): 387. DOI: 10.1200/jco.2019.37.4_suppl.387. [54] VILLANUEVA L, LWIN Z, CHUNG HC, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study[J]. J Clin Oncol, 2021, 39( 3_suppl): 321. DOI: 10.1200/jco.2021.39.3_suppl.321. [55] BURRIS HA 3, OKUSAKA T, VOGEL A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer(TOPAZ-1): Patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2024, 25( 5): 626- 635. DOI: 10.1016/S1470-2045(24)00082-2. [56] KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/S0140-6736(23)00727-4. [57] JIAN Z, FAN J, SHI GM, et al. Gemox chemotherapy in combination with anti-PD1 antibody toripalimab and lenvatinib as first-line treatment for advanced intrahepatic cholangiocarcinoma: A phase 2 clinical trial[J]. J Clin Oncol, 2021, 39( 15_suppl): 4094. DOI: 10.1200/jco.2021.39.15_suppl.4094. [58] WANG K, LIU ZH, YU HM, et al. Efficacy and safety of a triple combination of atezolizumab, bevacizumab plus GEMOX for advanced biliary tract cancer: A multicenter, single-arm, retrospective study[J]. Therap Adv Gastroenterol, 2023, 16: 17562848231160630. DOI: 10.1177/17562848231160630. [59] SHIRAHAMA T, MUROYA D, MATSUEDA S, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer[J]. Cancer Sci, 2017, 108( 5): 838- 845. DOI: 10.1111/cas.13193. [60] GOLDSTEIN D, LEMECH C, VALLE J. New molecular and immunotherapeutic approaches in biliary cancer[J]. ESMO Open, 2017, 2( Suppl 1): e000152. DOI: 10.1136/esmoopen-2016-000152. [61] FENG KC, LIU Y, GUO YL, et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers[J]. Protein Cell, 2018, 9( 10): 838- 847. DOI: 10.1007/s13238-017-0440-4. [62] TRAN E, TURCOTTE S, GROS A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer[J]. Science, 2014, 344( 6184): 641- 645. DOI: 10.1126/science.1251102. -



 PDF下载 ( 922 KB)
PDF下载 ( 922 KB)

 下载:
下载: