2型糖尿病胰腺癌裸鼠模型的建立及活体成像观察
DOI: 10.12449/JCH240625
Establishment and in vivo imaging observation of a nude mouse model of type 2 diabetes mellitus and pancreatic cancer
-
摘要:
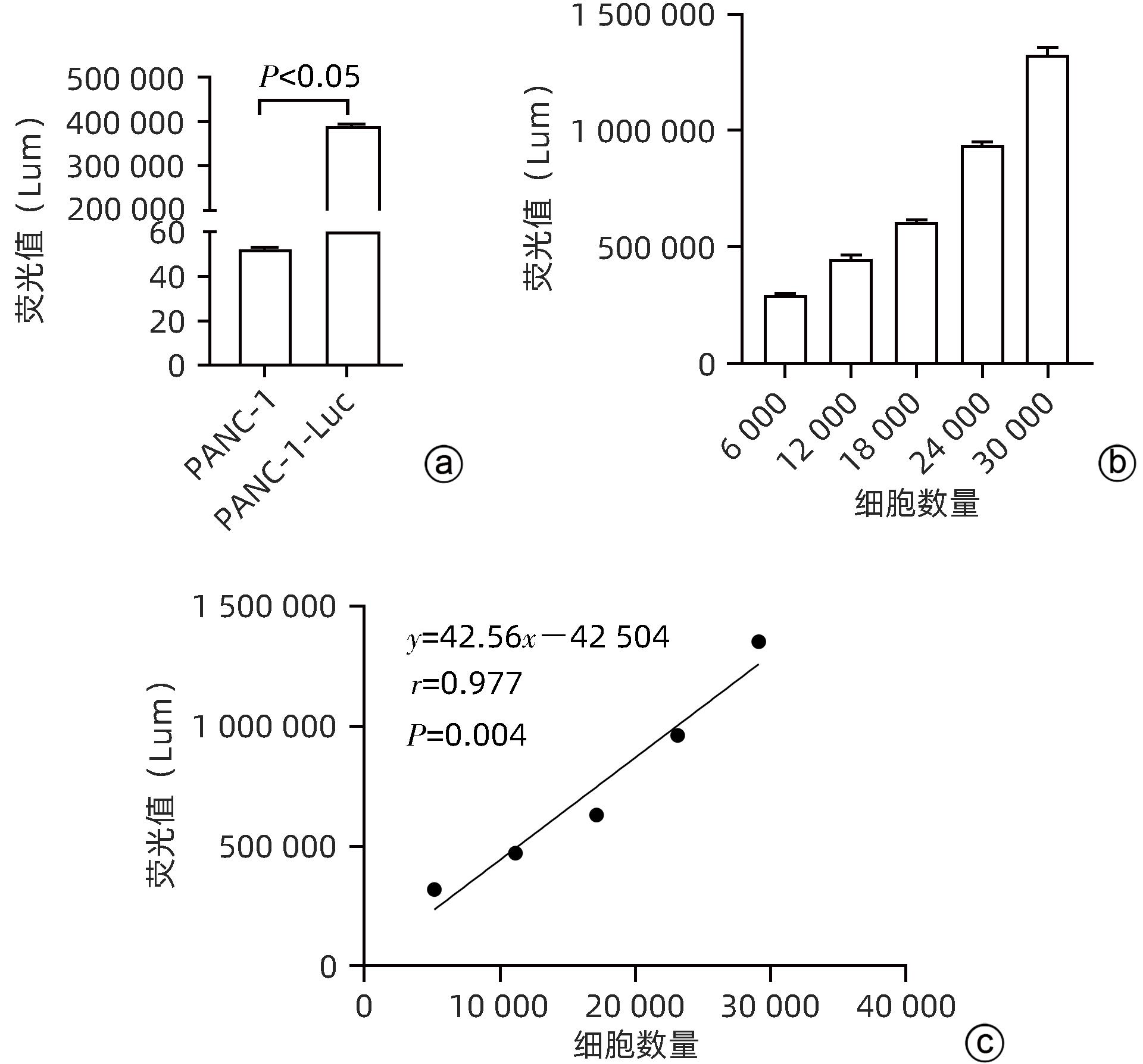
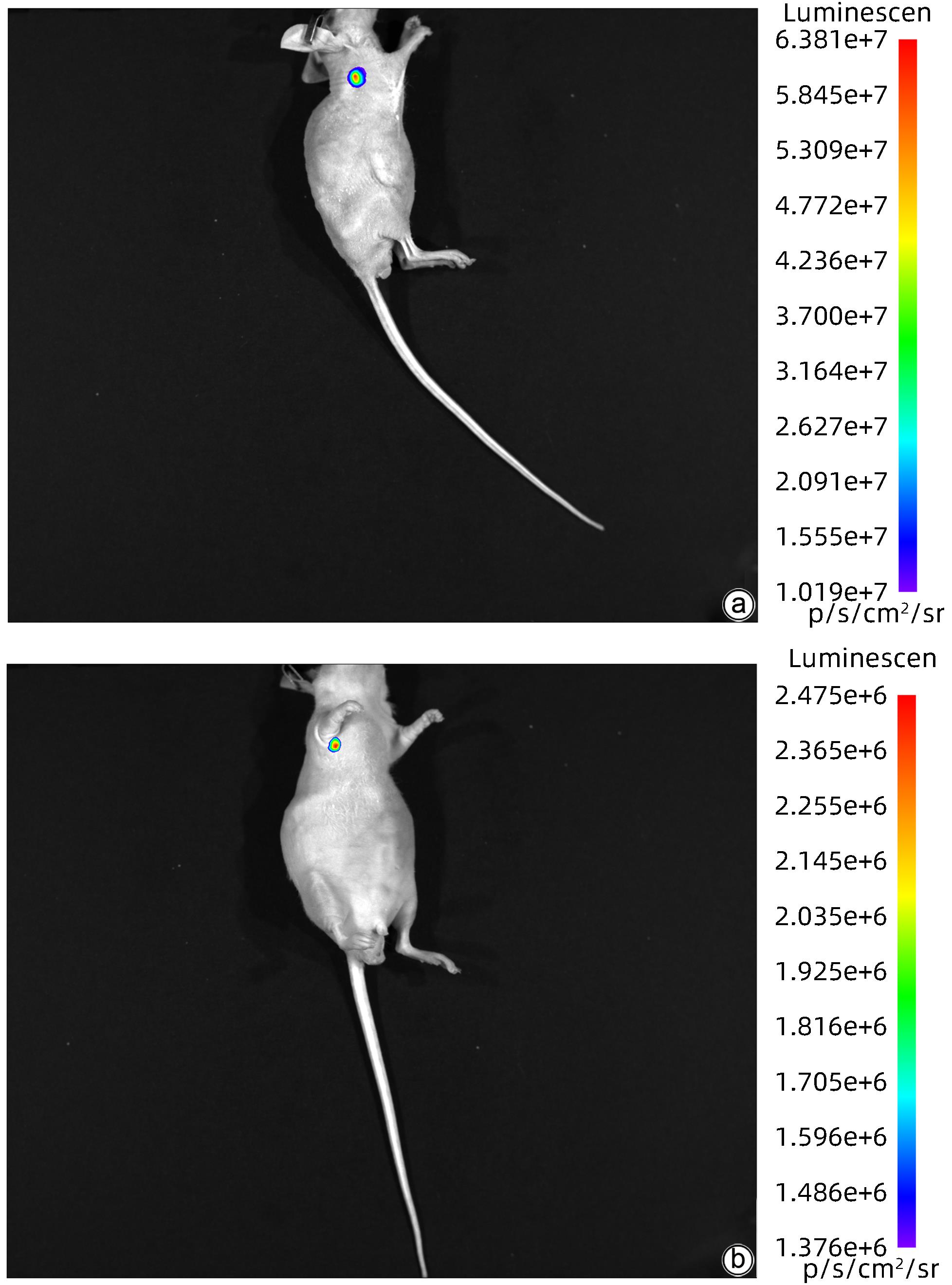
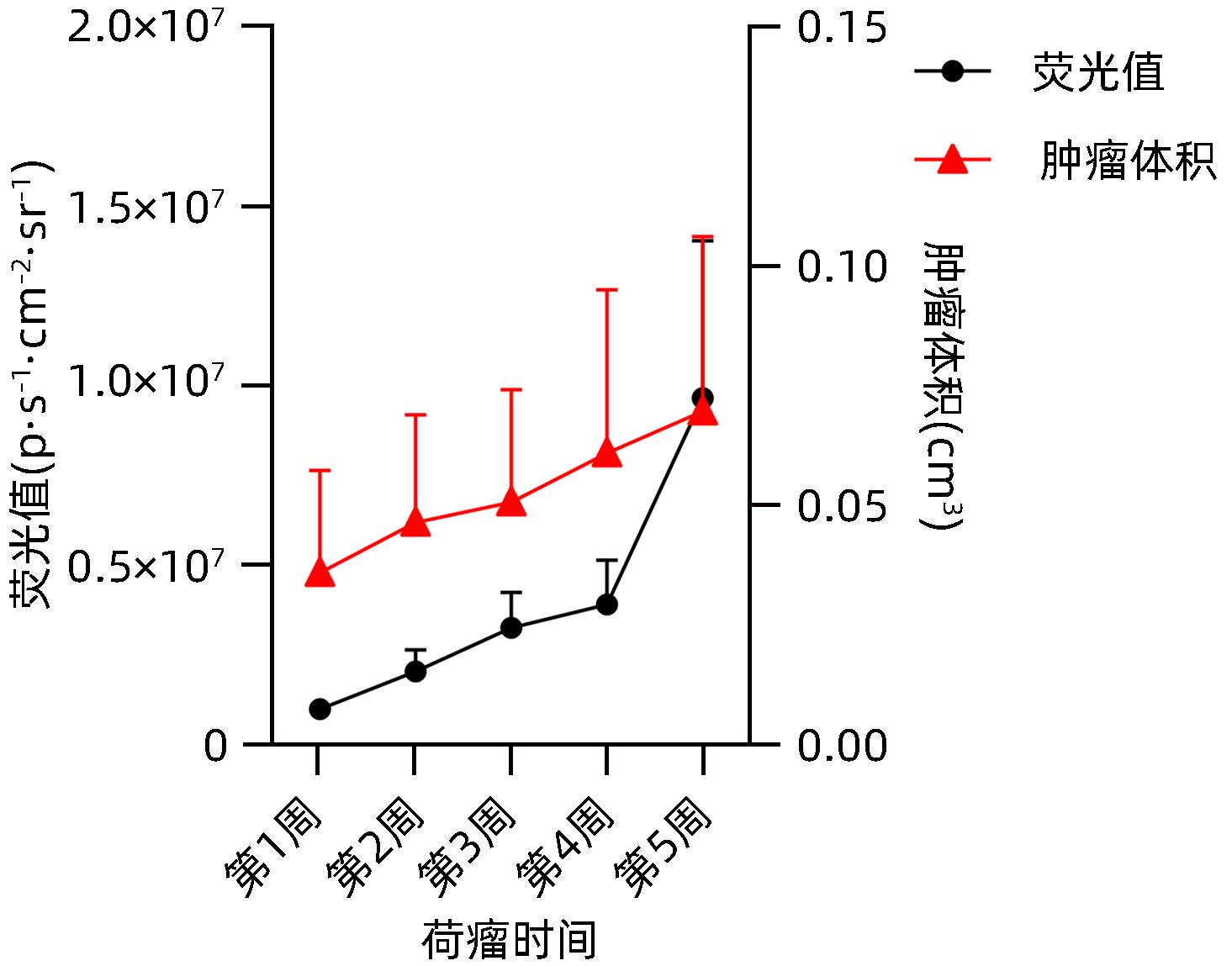
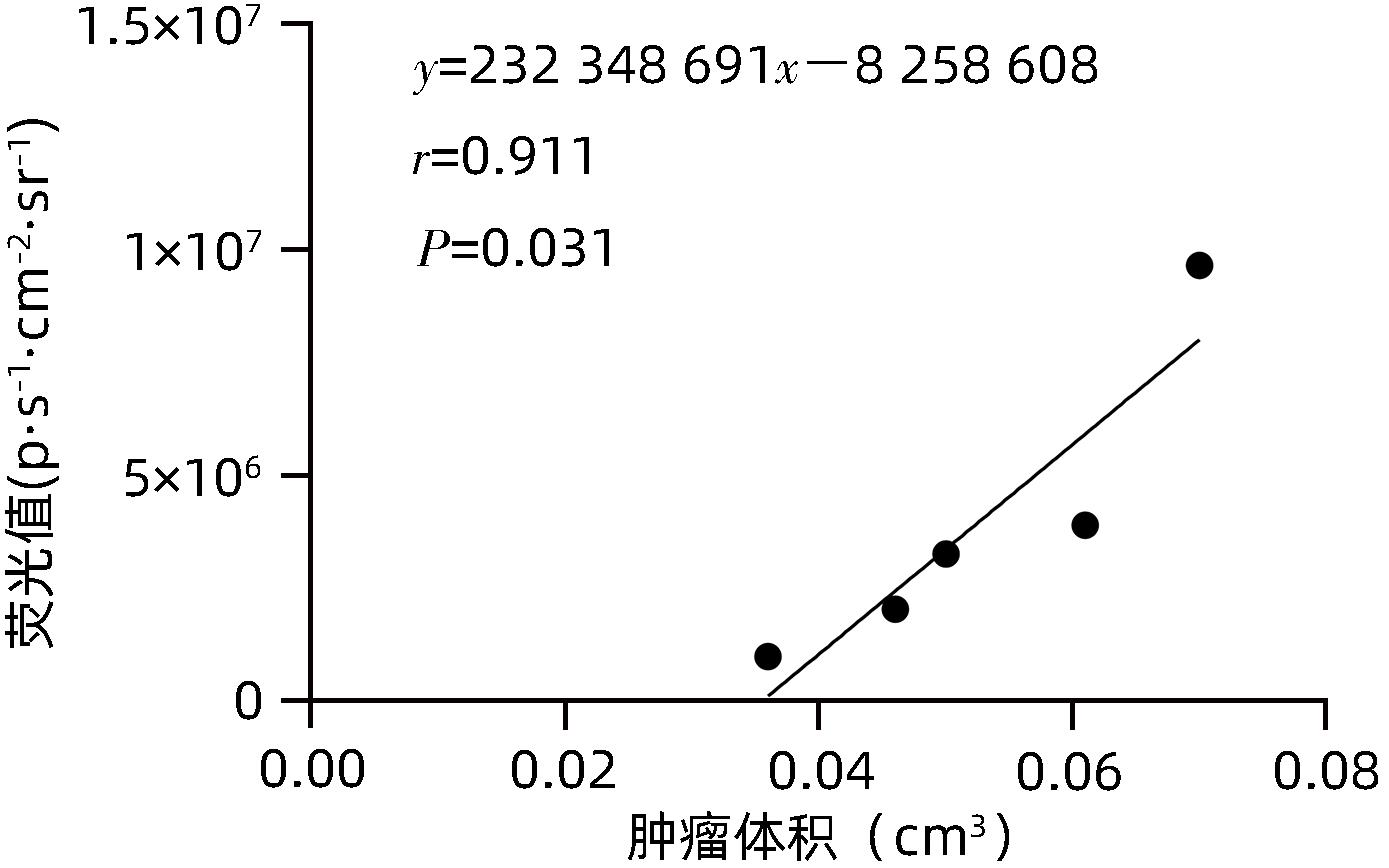
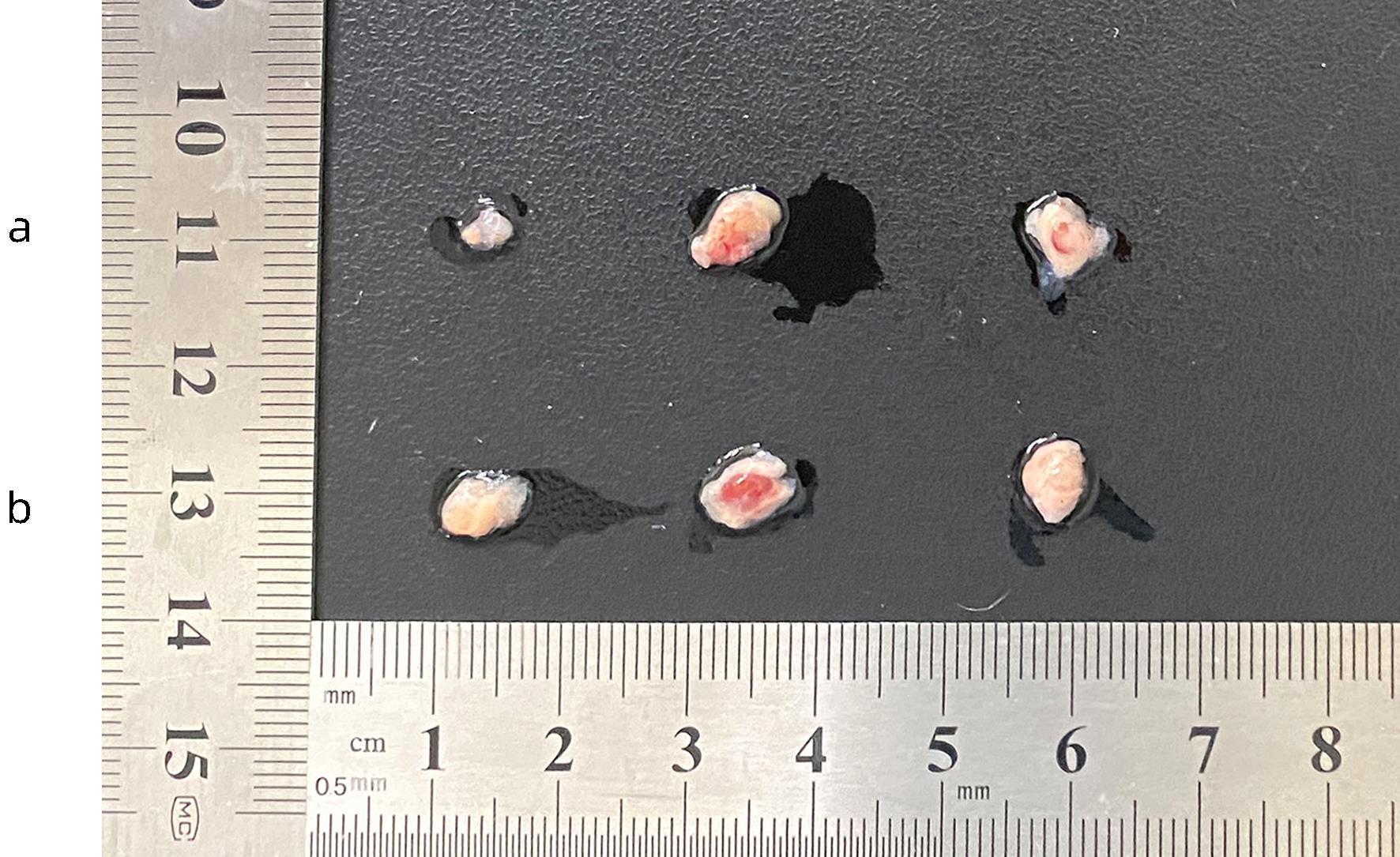
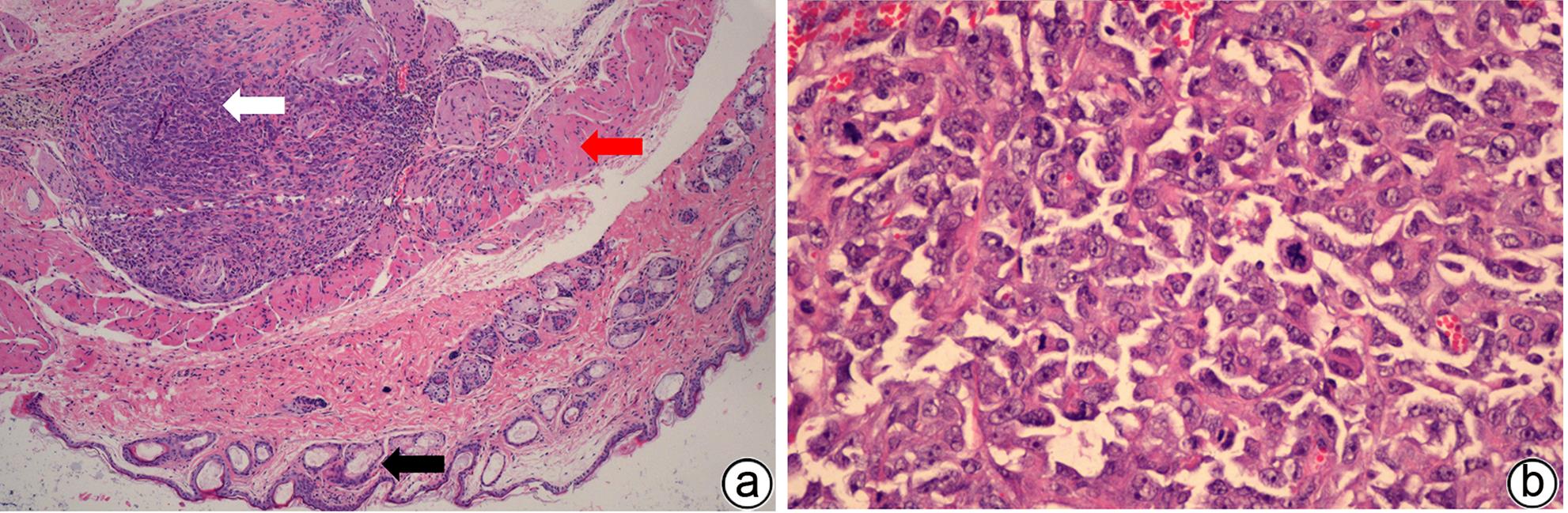
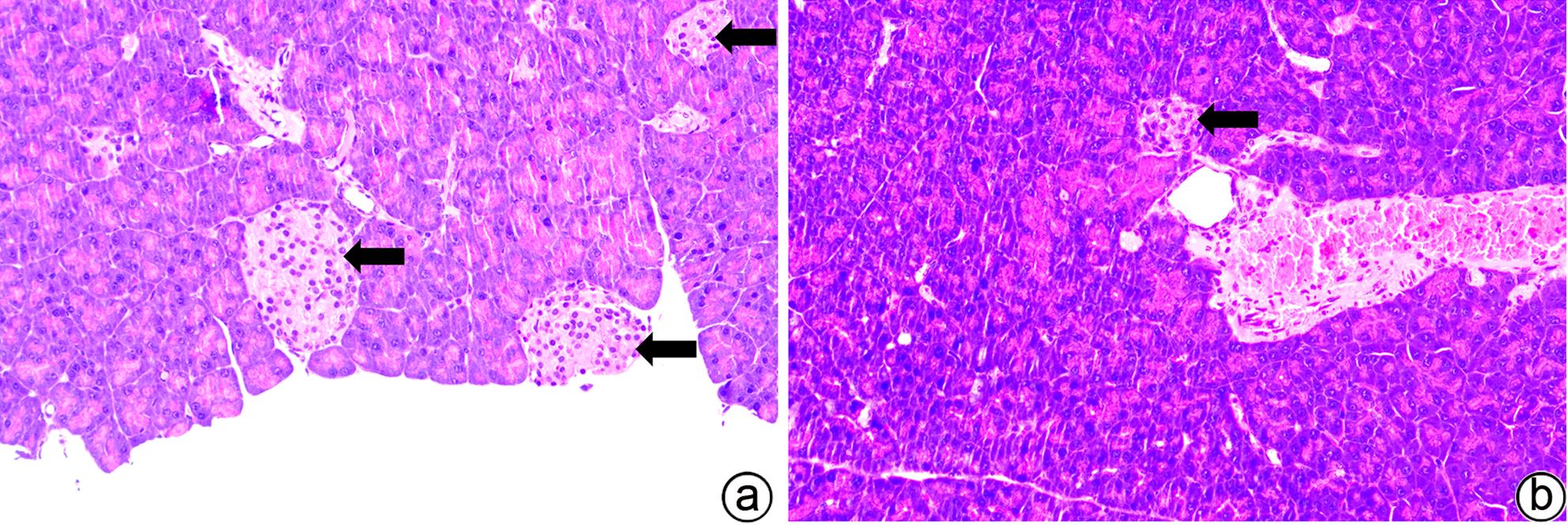
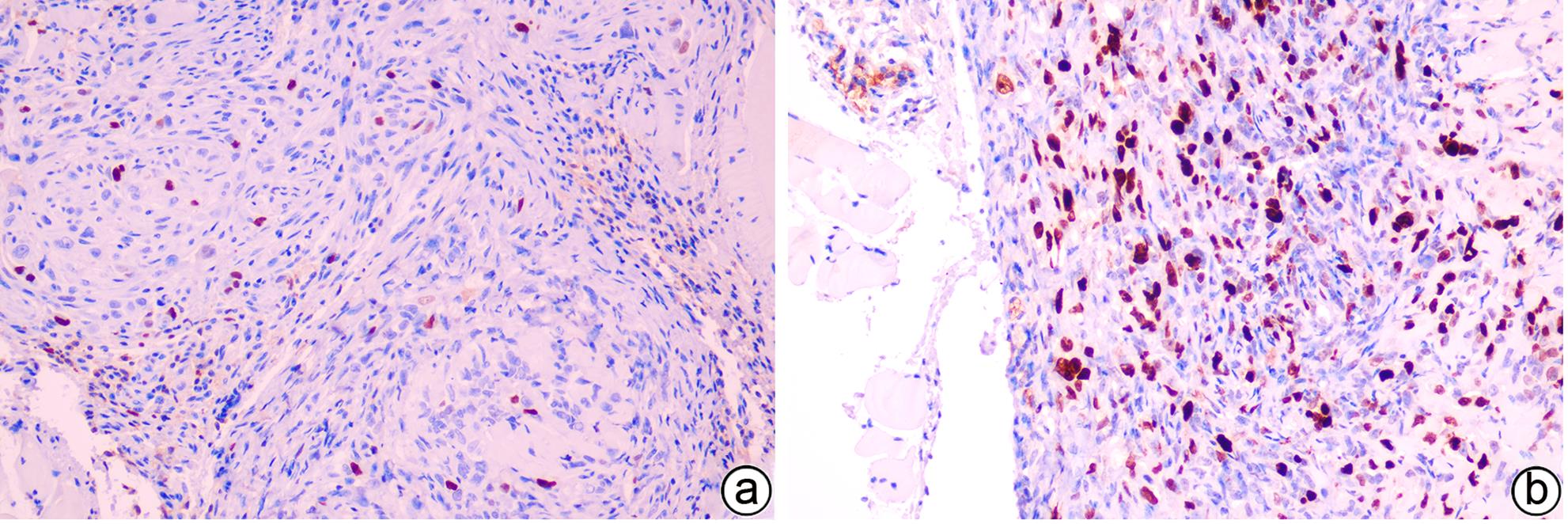
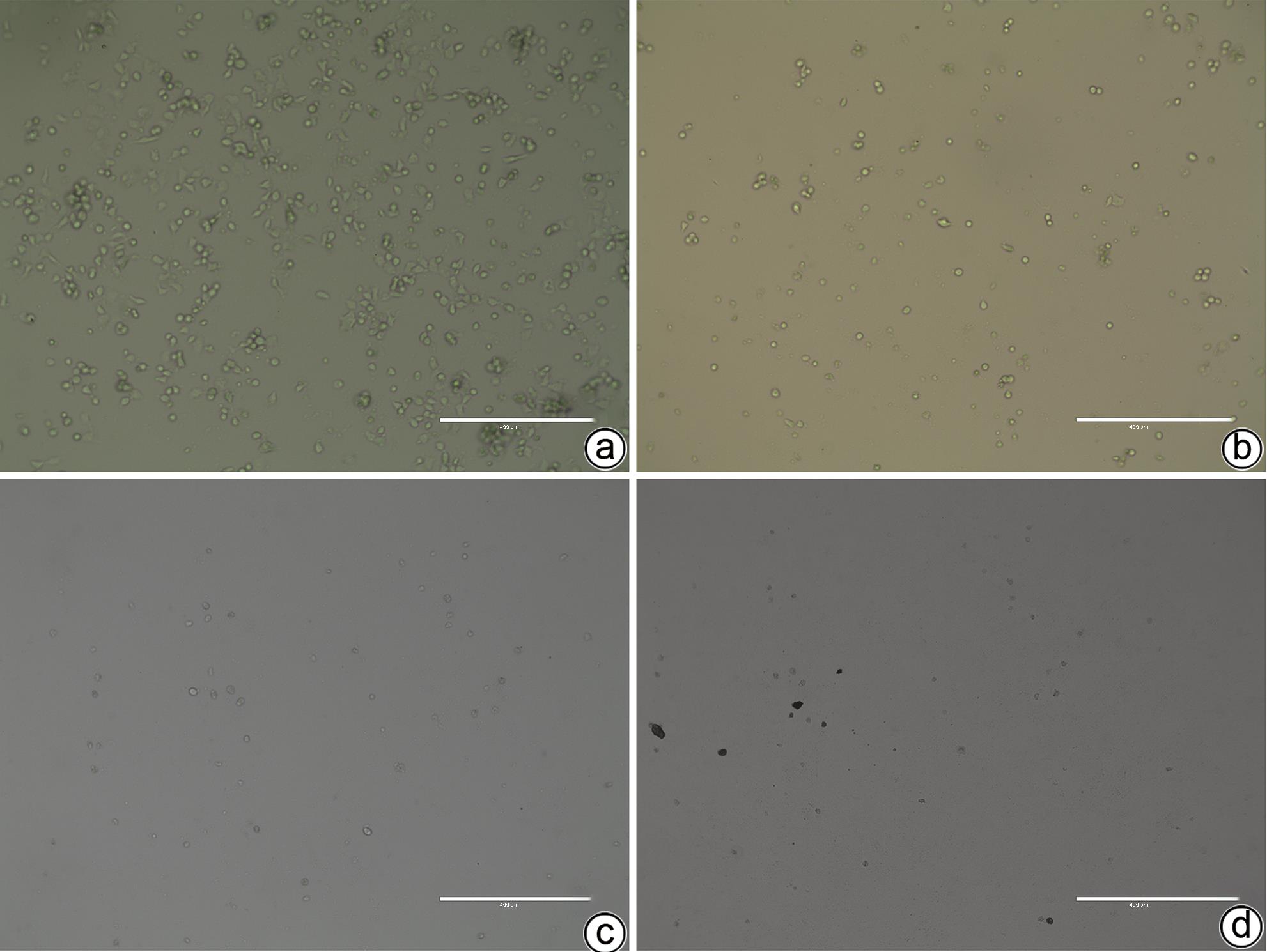
目的 建立可动态观察成瘤过程并进行体内研究的2型糖尿病(T2DM)胰腺癌裸鼠模型。 方法 首先,通过慢病毒载体GV260转染人胰腺癌细胞(PANC-1细胞)构建能稳定表达萤火虫荧光素酶的胰腺癌细胞株(PANC-1-Luc细胞)。然后,将36只SPF级裸鼠随机分为对照组(n=12,血糖正常的胰腺癌裸鼠)和模型组(n=24,T2DM胰腺癌裸鼠)。对照组:先给予繁殖饲料喂养,之后将PANC-1-Luc细胞异位种植于裸鼠皮下;模型组:先给予高脂饲料喂养联合腹腔注射1% STZ,之后将PANC-1-Luc细胞异位种植于裸鼠皮下。用荧光活体成像系统和人工测量法同步动态监测2组裸鼠胰腺癌生长情况,绘制肿瘤生长曲线、分析荧光值与肿瘤体积的关系。显微镜下观察裸鼠皮下肿瘤及胰岛,验证造模是否成功;同时,通过免疫组化检测肿瘤组织Ki-67的表达来分析高血糖对裸鼠胰腺癌生长的影响。正态分布计量资料组间比较采用成组t检验,非正态分布计量资料组间比较采用Mann-Whitney U检验。 结果 确定PANC-1细胞慢病毒载体稳定转染的最佳病毒滴度为5×107 TU/mL,用嘌呤霉素筛选的最佳浓度为20 μg/mL、最佳筛选时间为9天;PANC-1-Luc细胞的荧光值与细胞数量呈线性正相关,线性方程为y=42.56x-42 504(r=0.977,P=0.004)。T2DM裸鼠模型血糖值为23.05(19.25~26.40)mmol/L,且每只裸鼠的血糖均高于11.1 mmol/L,与对照组裸鼠血糖值[6.15(5.20~7.30)mmol/L]相比,差异有统计学意义(Z=-8.45,P<0.001)。与对照组相比,模型组胰腺组织内胰岛数量减少、体积减小、形状不规则、边界模糊,同时移植瘤病理学检查确认镜下为胰腺癌组织,可判定T2DM裸鼠胰腺癌模型造模成功。模型组皮下肿瘤大小与荧光值呈线性正相关,线性方程为y=232 348 691x-8 258 608(r=0.911,P=0.031);模型组移植瘤Ki-67免疫组化阳性率显著高于对照组[(50.333±7.808)% vs (15.917±4.055)%,t=13.55,P<0.001],说明模型组肿瘤增殖较快。 结论 本研究所构建的T2DM裸鼠胰腺癌模型可模拟T2DM背景下胰腺癌发生、发展的病理过程,动态观察高血糖对体内胰腺癌细胞生长的影响,从而为T2DM背景下胰腺癌发生、发展的体内研究提供新的实验载体。 Abstract:Objective To establish a nude mouse model of type 2 diabetes mellitus (T2DM) and pancreatic cancer that allows dynamic observation of tumor formation process and facilitates in vivo research. Methods At first, human pancreatic cancer PANC-1 cells were transfected with lentiviral vector GV260 to construct the pancreatic cancer cell line PANC-1-Luc with stable expression of firefly luciferase. Then, 36 specific pathogen-free nude mice were randomly divided into control group with 12 mice and model group with 24 mice (nude mice with T2DM and pancreatic cancer). The mice in the control group were fed with breeding diet and were then given ectopic subcutaneous implantation of PANC-1-Luc cells, and those in the model group were first given high-fat diet and intraperitoneal injection of 1% STZ, followed by ectopic subcutaneous implantation of PANC-1-Luc cells. The fluorescence in vivo imaging system and the manual measurement method were used for simultaneous and dynamic monitoring of the growth of pancreatic cancer in nude mice in the two groups, and the tumor growth curve was plotted to investigate the correlation between fluorescence value and tumor volume. Subcutaneous tumors and pancreatic islets were observed under a microscope to verify whether the model was successfully established, and immunohistochemistry was used to measure the expression of Ki-67 in tumor tissue to investigate the influence of hyperglycemia on the growth of pancreatic cancer in nude mice. The independent-samples t test was used for comparison of normally distributed continuous data between groups, and the Mann-Whitney U test was used for comparison of non-normally distributed continuous data between groups. Results The optimal virus titer was determined as 5×107 TU/mL for the stable transfection of lentiviral vector in PANC-1 cells, and the optimal concentration selected with puromycin was 20 μg/mL, with an optimal selection time of 9 days. The fluorescence value of PANC-1-Luc cells was linearly and positively correlated with the number of cells, with the linear equation of y=42.56x-42 504 (r=0.977, P=0.004). The blood glucose value of T2DM nude mice was 23.05 (19.25 — 26.40) mmol/L, with a blood glucose level of >11.1 mmol/L in each nude mouse, and there was a significant difference in blood glucose value between the T2DM nude mice and the control nude [6.15 (5.20 — 7.30) mmol/L] (Z=-8.45, P<0.001). Compared with the control group, the model group had reductions in the number and volume of pancreatic islets, with irregular shapes and unclear boundaries, and pathological examination confirmed that the xenograft tumor was pancreatic cancer tissue, which showed that the model was established successfully. In the model group, there was a linear positive correlation between subcutaneous tumor size and fluorescence values, with the linear equation of y=232 348 691x-8 258 608 (r=0.911, P=0.031). The model group had a significantly higher positive rate of Ki-67 than the control group (50.333%± 7.808% vs 15.917%±4.055%, t=13.55, P<0.001), suggesting rapid tumor proliferation in the model group. Conclusion The T2DM nude mouse model of pancreatic cancer established in this study can simulate the pathological process of the development and progression of pancreatic cancer in the context of T2DM and dynamically observe the influence of hyperglycemia on the growth of pancreatic cancer cells in vivo, thereby providing a new experimental vector for the in vivo study of the development and progression of pancreatic cancer in the context of T2DM. -
Key words:
- Diabetes Mellitus, Type 2 /
- Pancreatic Neoplasms /
- Disease Models, Animal
-
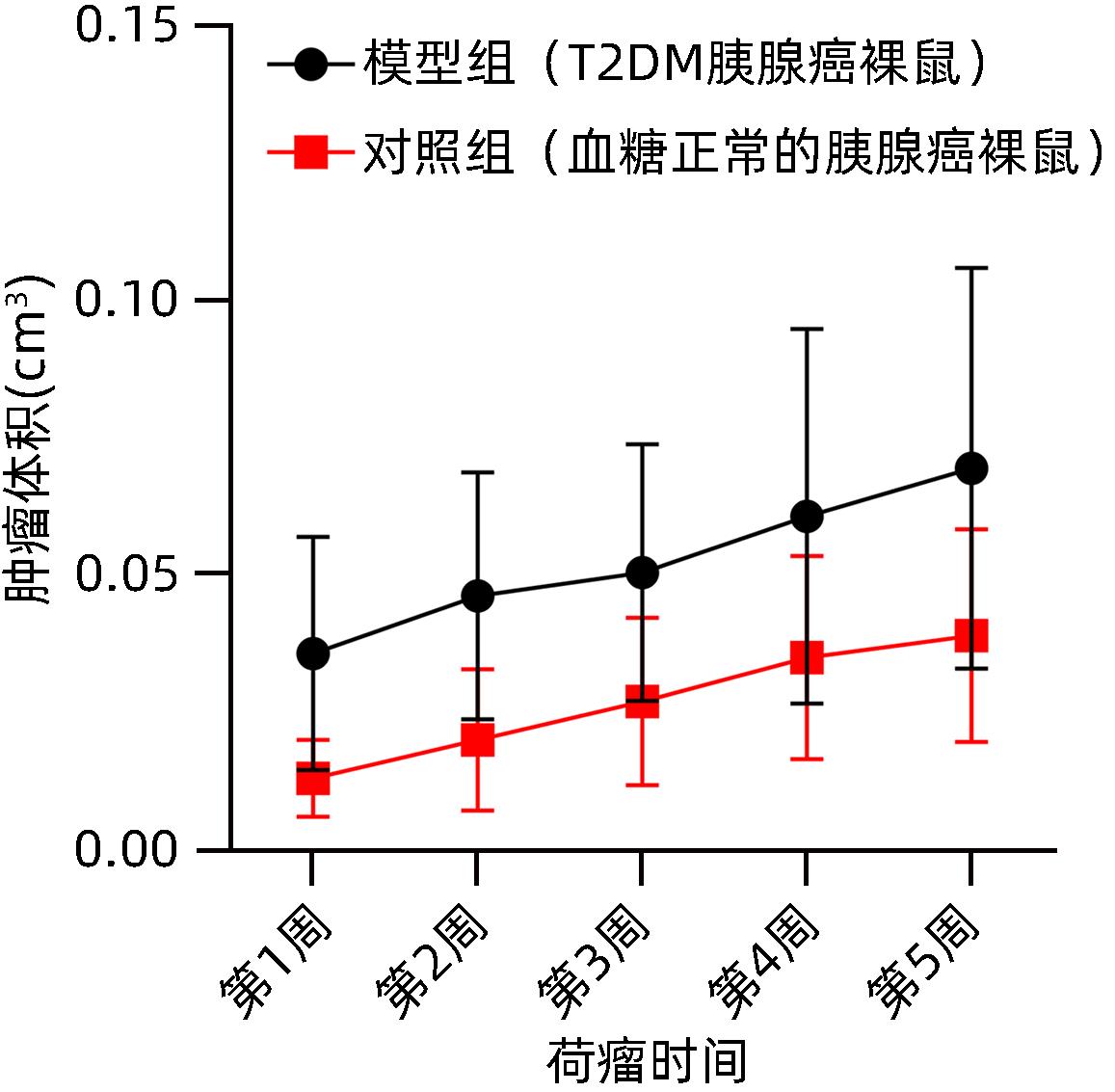
表 1 模型组及对照组裸鼠皮下肿瘤大小变化(cm3)
Table 1. Changes in subcutaneous tumor size of nude mice in the model group and the control group (cm3)
时间 对照组(n=12) 模型组(n=24) t值 P值 第1周 0.013±0.007 0.036±0.021 3.535 0.004 第2周 0.020±0.012 0.046±0.022 3.490 0.002 第3周 0.027±0.015 0.051±0.023 3.490 0.002 第4周 0.035±0.019 0.061±0.034 2.285 0.035 第5周 0.039±0.019 0.070±0.037 2.549 0.018 表 2 模型组及对照组裸鼠皮下肿瘤荧光值变化(p⋅s-1⋅cm-2⋅sr-1)
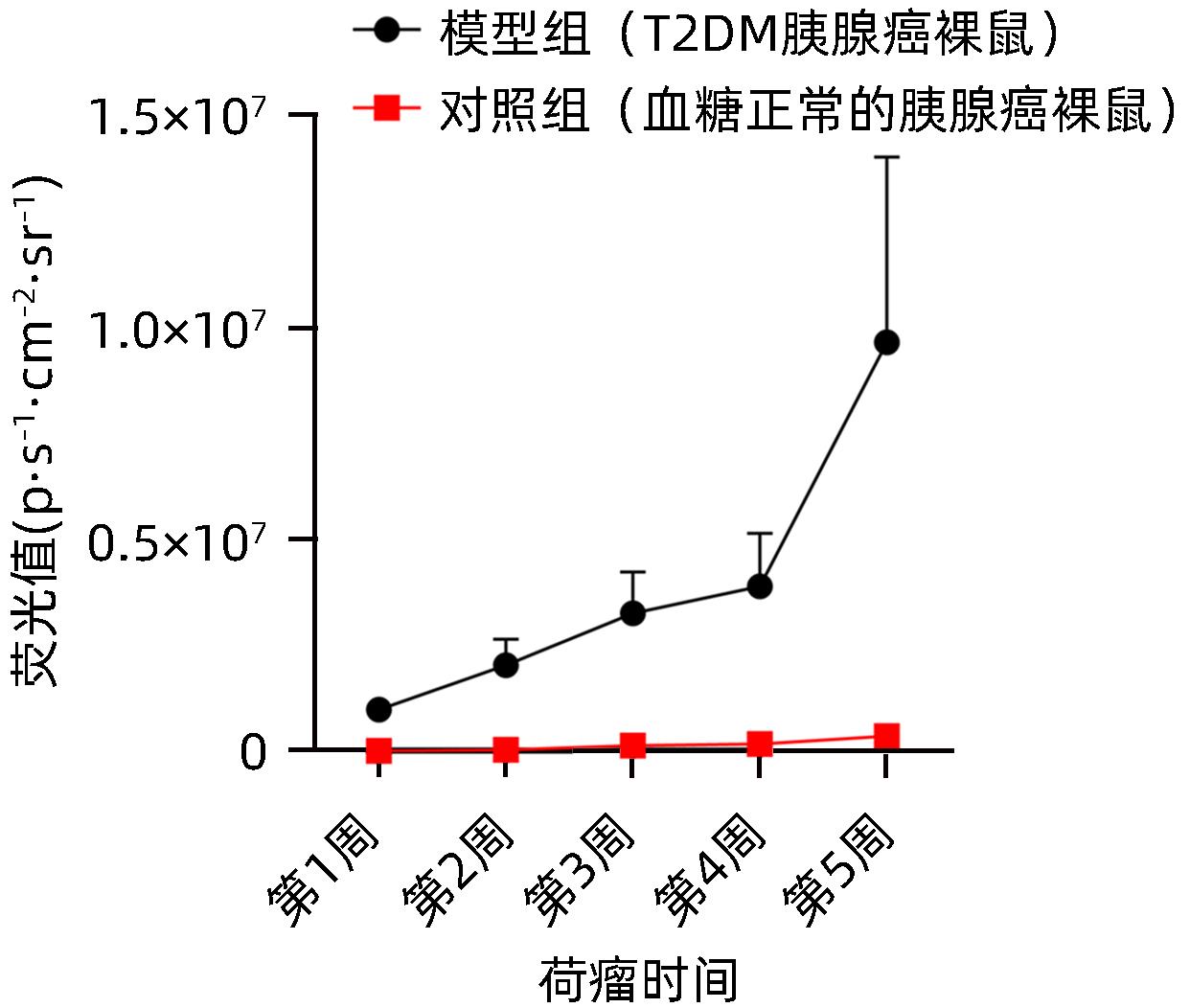
Table 2. Changes in subcutaneous tumor values of nude mice in the model group and the control group (p·s-1·cm-2·sr-1)
时间 对照组(n=12) 模型组(n=24) t值 P值 第1周 6 460.83±4 200.33 985 416.67±97 565.32 34.726 <0.001 第2周 28 169.17±20 112.81 2 029 250.00±612 538.25 11.311 <0.001 第3周 131 601.67±101 868.71 3 251 666.67±981 546.40 10.953 <0.001 第4周 174 560.00±133 203.77 3 897 500.00±1 248 631.07 10.270 <0.001 第5周 359 734.17±236 685.20 9 650 833.33±4 384 569.45 7.330 <0.001 -
[1] SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71( 3): 209- 249. DOI: 10.3322/caac.21660. [2] XIE ZB, GAO Y, HO C, et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment[J]. Gut, 2022, 71( 3): 568- 579. DOI: 10.1136/gutjnl-2020-323014. [3] BOSETTI C, ROSATO V, LI D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: An analysis from the International Pancreatic Cancer Case-Control Consortium[J]. Ann Oncol, 2014, 25( 10): 2065- 2072. DOI: 10.1093/annonc/mdu276. [4] LI WJ, ZHANG XH, SANG H, et al. Effects of hyperglycemia on the progression of tumor diseases[J]. J Exp Clin Cancer Res, 2019, 38( 1): 327. DOI: 10.1186/s13046-019-1309-6. [5] NOH Y, JEON SM, SHIN S. Association between glucose-lowering treatment and cancer metastasis among patients with preexisting type 2 diabetes and incident malignancy[J]. Int J Cancer, 2019, 144( 7): 1530- 1539. DOI: 10.1002/ijc.31870. [6] OVERBEEK JA, van HERK-SUKEL MPP, VISSERS PAJ, et al. Type 2 diabetes, but not insulin(analog) treatment, is associated with more advanced stages of breast cancer: A national linkage of cancer and pharmacy registries[J]. Diabetes Care, 2019, 42( 3): 434- 442. DOI: 10.2337/dc18-2146. [7] POPOVIC K, SMOLOVIĆ B, MARTINOVIĆ M, et al. The relationship between diabetes mellitus and pancreatic cancer-diabetes mellitus as a red flag for pancreatic cancer[J]. Cancer Epidemiol Biomarkers Prev, 2023, 32( 3): 298- 305. DOI: 10.1158/1055-9965.EPI-22-0951. [8] LI JH, MA JG, HAN L, et al. Hyperglycemic tumor microenvironment induces perineural invasion in pancreatic cancer[J]. Cancer Biol Ther, 2015, 16( 6): 912- 921. DOI: 10.1080/15384047.2015.1040952. [9] LI W, ZHANG L, CHEN X, et al. Hyperglycemia promotes the epithelial-mesenchymal transition of pancreatic cancer via hydrogen peroxide[J]. Oxid Med Cell Longev, 2016, 2016: 5190314. DOI: 10.1155/2016/5190314. [10] CHENG L, QIN T, MA JG, et al. Hypoxia-inducible factor-1α mediates hyperglycemia-induced pancreatic cancer glycolysis[J]. Anticancer Agents Med Chem, 2019, 19( 12): 1503- 1512. DOI: 10.2174/1871520619666190626120359. [11] ZHANG L, ZHANG WN, ZHANG X, et al. High-glucose microenvironment promotes perineural invasion of pancreatic cancer via activation of hypoxia inducible factor 1α[J]. Oncol Rep, 2022, 47( 4): 64. DOI: 10.3892/or.2022.8275. [12] LI W, LIU H, QIAN WK, et al. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer[J]. Comput Struct Biotechnol J, 2018, 16: 479- 487. DOI: 10.1016/j.csbj.2018.10.006. [13] DEL PUERTO-NEVADO L, MINGUEZ P, CORTON M, et al. Molecular evidence of field cancerization initiated by diabetes in colon cancer patients[J]. Mol Oncol, 2019, 13( 4): 857- 872. DOI: 10.1002/1878-0261.12438. [14] KANG J, LI CQ, GAO XH, et al. Metformin inhibits tumor growth and affects intestinal flora in diabetic tumor-bearing mice[J]. Eur J Pharmacol, 2021, 912: 174605. DOI: 10.1016/j.ejphar.2021.174605. [15] JIANG YG, FENG CX, SHI YH, et al. Eugenol improves high-fat diet/streptomycin-induced type 2 diabetes mellitus(T2DM) mice muscle dysfunction by alleviating inflammation and increasing muscle glucose uptake[J]. Front Nutr, 2022, 9: 1039753. DOI: 10.3389/fnut.2022.1039753. [16] CUI XN, FENG J, WEI TJ, et al. Pancreatic alpha cell glucagon-liver FGF21 axis regulates beta cell regeneration in a mouse model of type 2 diabetes[J]. Diabetologia, 2023, 66( 3): 535- 550. DOI: 10.1007/s00125-022-05822-2. [17] ELSNER M, GULDBAKKE B, TIEDGE M, et al. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin[J]. Diabetologia, 2000, 43( 12): 1528- 1533. DOI: 10.1007/s001250051564. [18] HE CX, WANG K, XIA J, et al. Natural exosomes-like nanoparticles in mung bean sprouts possesses anti-diabetic effects via activation of PI3K/Akt/GLUT4/GSK-3β signaling pathway[J]. J Nanobiotechnology, 2023, 21( 1): 349. DOI: 10.1186/s12951-023-02120-w. [19] MCNEILLY AD, GALLAGHER JR, EVANS ML, et al. Chronic hyperglycaemia increases the vulnerability of the hippocampus to oxidative damage induced during post-hypoglycaemic hyperglycaemia in a mouse model of chemically induced type 1 diabetes[J]. Diabetologia, 2023, 66( 7): 1340- 1352. DOI: 10.1007/s00125-023-05907-6. [20] ENTENBERG D, OKTAY MH, CONDEELIS JS. Intravital imaging to study cancer progression and metastasis[J]. Nat Rev Cancer, 2023, 23( 1): 25- 42. DOI: 10.1038/s41568-022-00527-5. [21] SYED AJ, ANDERSON JC. Applications of bioluminescence in biotechnology and beyond[J]. Chem Soc Rev, 2021, 50( 9): 5668- 5705. DOI: 10.1039/d0cs01492c. [22] LIU S, SU YC, LIN MZ, et al. Brightening up biology: Advances in luciferase systems for in vivo imaging[J]. ACS Chem Biol, 2021, 16( 12): 2707- 2718. DOI: 10.1021/acschembio.1c00549. [23] YAN YC, SHI PF, SONG WL, et al. Chemiluminescence and bioluminescence imaging for biosensing and therapy: in vivo and in vivo perspectives[J]. Theranostics, 2019, 9( 14): 4047- 4065. DOI: 10.7150/thno.33228. [24] MERLE N, ELMSHÄUSER S, STRASSHEIMER F, et al. Monitoring autochthonous lung tumors induced by somatic CRISPR gene editing in mice using a secreted luciferase[J]. Mol Cancer, 2022, 21( 1): 191. DOI: 10.1186/s12943-022-01661-2. [25] LI SF, RUAN ZY, ZHANG H, et al. Recent achievements of bioluminescence imaging based on firefly luciferin-luciferase system[J]. Eur J Med Chem, 2021, 211: 113111. DOI: 10.1016/j.ejmech.2020.113111. [26] DEROOSE CM, REUMERS V, GIJSBERS R, et al. Noninvasive monitoring of long-term lentiviral vector-mediated gene expression in rodent brain with bioluminescence imaging[J]. Mol Ther, 2006, 14( 3): 423- 431. DOI: 10.1016/j.ymthe.2006.05.007. [27] TAO ZN, LI T, MA HW, et al. Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation[J]. Cell Death Dis, 2018, 9( 11): 1063. DOI: 10.1038/s41419-018-0957-3. [28] YOU K, WANG DJ, WANG L, et al. Effect of NOR1 gene knockout on nude mice xenograft tumor of human liver cancer and its mechanism of action[J]. J Clin Hepatol, 2020, 36( 2): 381- 386. DOI: 10.3969/j.issn.1001-5256.2020.02.030.游焜, 王大军, 王亮, 等. 敲除NOR1基因对人肝癌裸鼠移植瘤的影响及作用机制[J]. 临床肝胆病杂志, 2020, 36( 2): 381- 386. DOI: 10.3969/j.issn.1001-5256.2020.02.030. [29] LI XY, YANG YF, CHEN Y, et al. Expression and significance of response gene to complement 32 in liver regeneration after partial hepatectomy in mice[J]. J Clin Hepatol, 2023, 39( 10): 2396- 2405. DOI: 10.3969/j.issn.1001-5256.2023.10.018.李兴元, 杨艳芳, 陈琰, 等. 补体应答基因32在小鼠部分肝切除后肝再生过程中的表达及意义[J]. 临床肝胆病杂志, 2023, 39( 10): 2396- 2405. DOI: 10.3969/j.issn.1001-5256.2023.10.018. [30] JANG SJ, KANG JH, KIM KI, et al. Application of bioluminescence imaging to therapeutic intervention of herpes simplex virus type I-Thymidine kinase/ganciclovir in glioma[J]. Cancer Lett, 2010, 297( 1): 84- 90. DOI: 10.1016/j.canlet.2010.04.028. [31] WANG XL, ROSOL M, GE SD, et al. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging[J]. Blood, 2003, 102( 10): 3478- 3482. DOI: 10.1182/blood-2003-05-1432. -



 PDF下载 ( 1970 KB)
PDF下载 ( 1970 KB)

 下载:
下载: