结直肠癌肿瘤相关成纤维细胞对免疫治疗和肝转移的影响
DOI: 10.12449/JCH240618
Impact of cancer-associated fibroblasts on immunotherapy and liver metastasis in colorectal cancer
-
摘要:
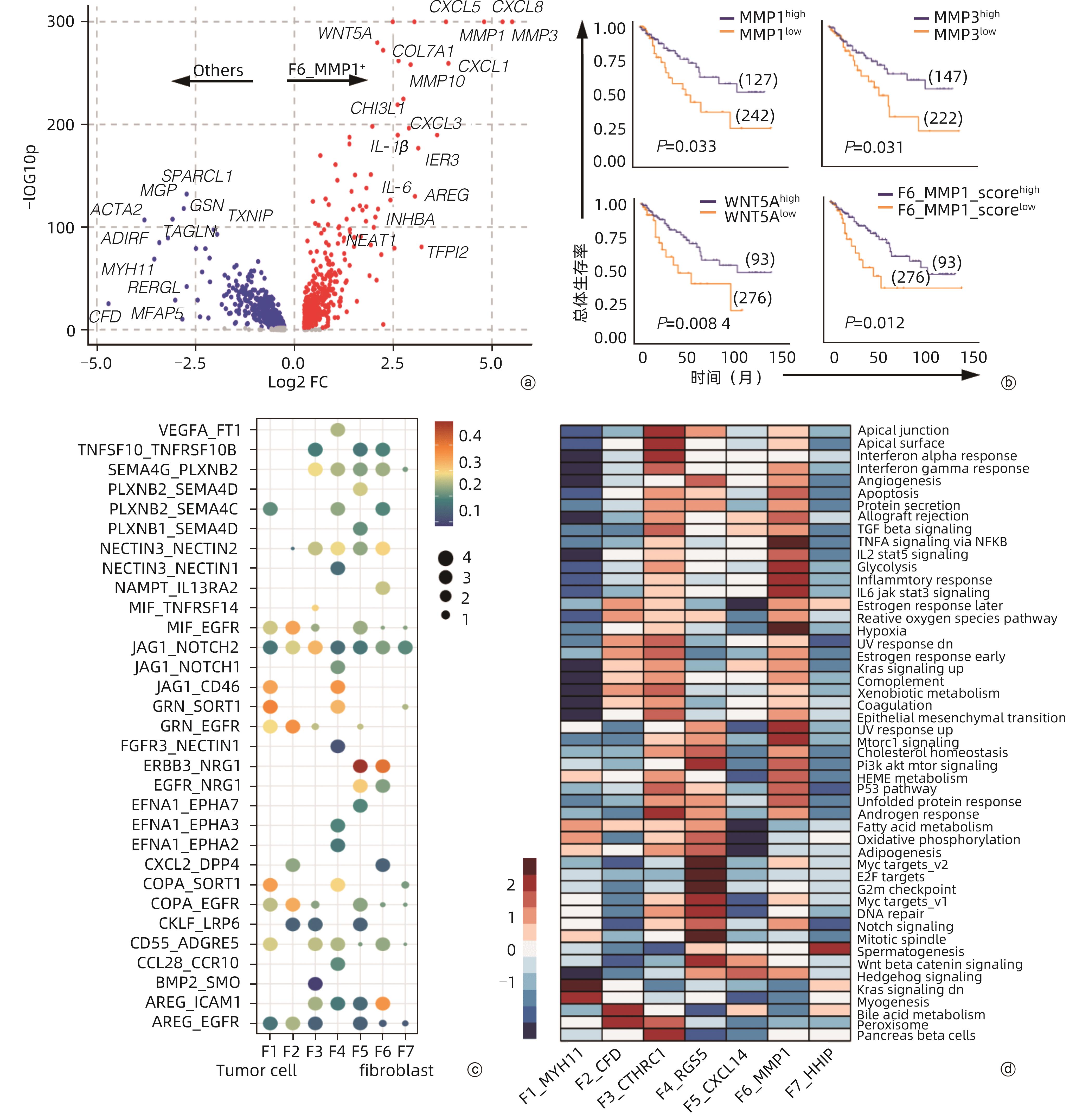
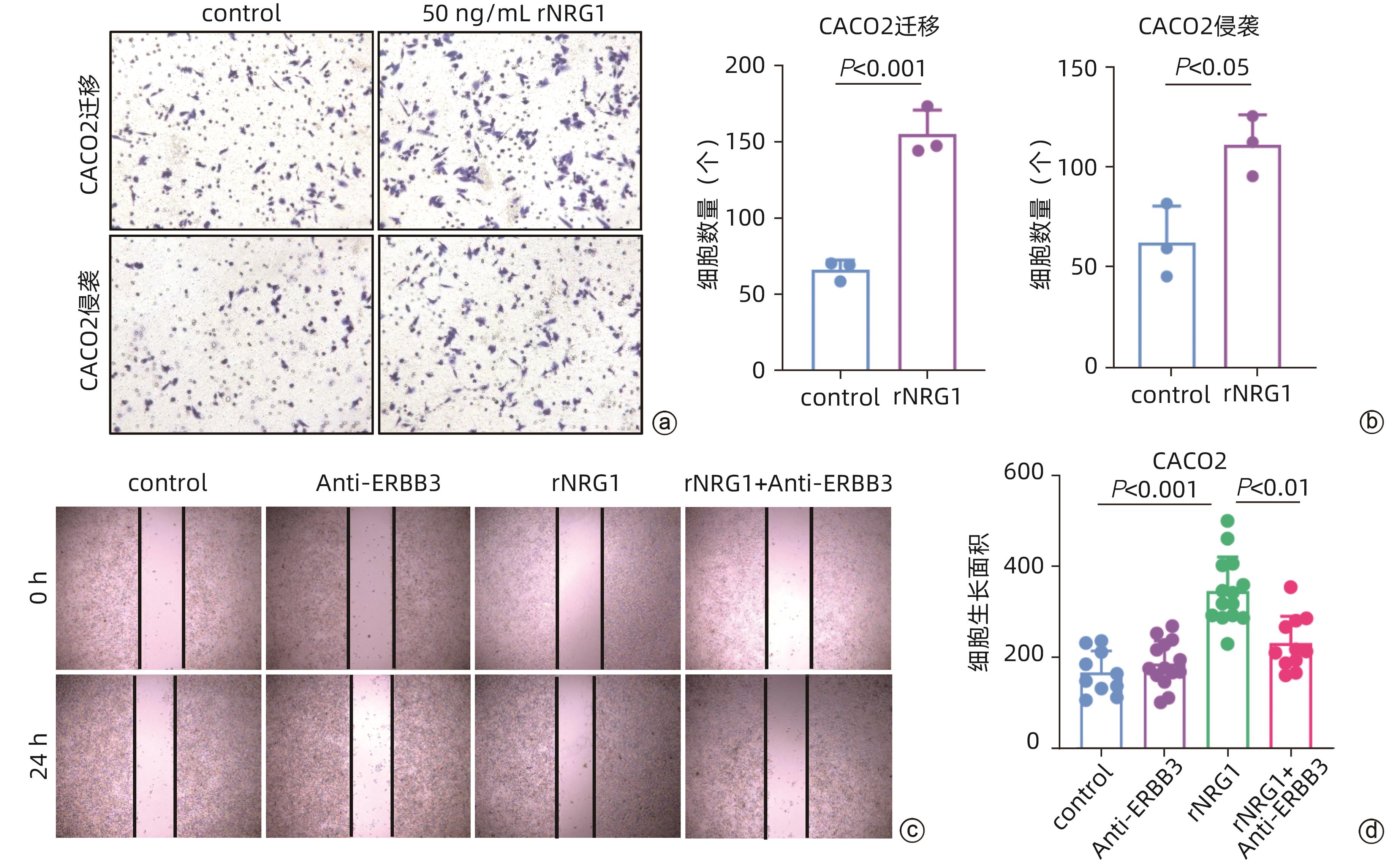
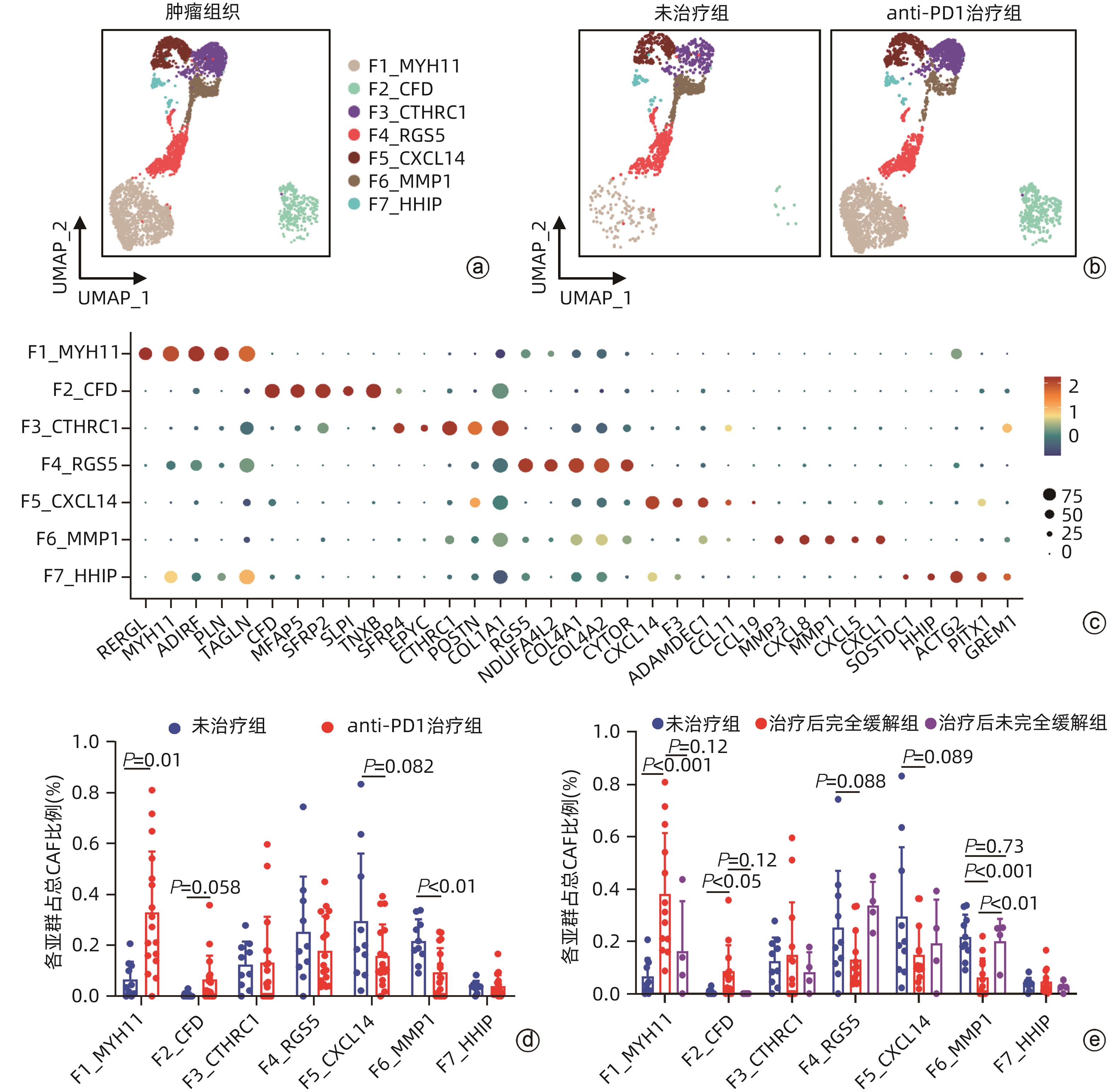
目的 探讨结直肠癌(CRC)中肿瘤相关成纤维细胞(CAF)对免疫治疗和肝转移的影响。 方法 从基因表达数据库(GEO)中下载错配修复缺陷(MMRd)的CRC患者相关的单细胞测序数据(GSE205506),利用R软件对原始测序数据进行预处理,建立CAF亚群降维图,并根据每个亚群的标志性基因对亚群进行命名,使用GraphPad对每种亚群的比例进行统计,分析CRC患者在程序性死亡受体1(PD-1)免疫治疗前后以及治疗后病理完全缓解(pCR)与病理未完全缓解(non-pCR)患者中具有明显差异的关键亚群,对关键亚群进行差异基因分析和基因通路富集分析,利用肿瘤基因组图谱(TCGA)数据库对关键CAF亚群的标志性基因进行预后生存分析,通过RNA测序数据对CRC肝转移患者原发灶中关键CAF亚群进行评分和比例计算。符合正态分布的计量资料两组间比较采用成组t检验,Kaplan-Meier法绘制生存曲线,Log-rank检验比较生存率。利用CellPhoneDB软件分析成纤维细胞亚群与肿瘤细胞间的受配体相互作用,并通过体外细胞实验验证关键配体分子NRG1对CRC细胞迁移侵袭能力的影响。 结果 CRC患者经过PD-1免疫治疗后,F6_MMP1+CAF比例减少(P<0.001),但这种减少只发生在免疫治疗后完全缓解的患者中,F6_MMP1+CAF与肿瘤迁移和侵袭相关的基因及信号通路表达上调,此外,F6_MMP1+CAF在CRC肝转移患者肿瘤组织中明显增多(P<0.000 1)。F6_MMP1+CAF表达的NRG1作为配体与肿瘤细胞表达的ERBB3受体相互作用,体外实验证明NRG1通过激活ERBB信号通路促进肿瘤细胞的迁移和侵袭(P<0.05)。 结论 F6_MMP1+CAF可能影响CRC患者PD-1免疫治疗的效果,并在促进CRC发生肝转移过程中发挥重要作用,F6_MMP1+CAF及产生的促肿瘤转移的NRG1或许可以作为潜在的CRC治疗靶点及预后标志物。 Abstract:Objective To investigate the impact of cancer-associated fibroblasts (CAFs) on immunotherapy and liver metastasis in colorectal cancer (CRC). Methods The single-cell sequencing data (GSE205506) of CRC patients with mismatch repair deficiency (MMRd) were downloaded from the gene expression omnibus database, and R software was used to preprocess the original sequencing data and establish the umap of fibroblast subpopulations, with each subpopulation named based on signature genes. GraphPad was used for the statistical analysis of the proportion of each fibroblast subpopulation, and the key subpopulations with significant differences were analyzed among CRC patients before and after PD-1 immunotherapy, as well as between the patients with pathological complete response (pCR) and those without pCR (non-pCR) after treatment. The analysis of differentially expressed genes and the gene pathway enrichment analysis were performed for the key subpopulations. The TCGA database was used to perform a prognostic and survival analysis of the signature genes of key CAF subpopulations, and RNA sequencing data were used to score and calculate the proportion of key CAF subpopulations in the primary lesions of CRC patients with liver metastasis. The independent-samples t test was used for comparison of normally distributed continuous data between two groups; the Kaplan-Meier method was used to plot survival curves, and the log-rank test was used to calculate survival rates. CellPhoneDB software was used to analyze the receptor-ligand interaction between fibroblast subpopulations and tumor cells, and in vitro cell experiments were used to validate the effect of NRG1, a key ligand molecule, on the migration and invasion abilities of CRC cells. Results After PD-1 immunotherapy for CRC patients, there was a significant reduction in the proportion of F6_MMP1+CAFs (P<0.001), which was only observed in patients achieving complete remission after immunotherapy. F6_MMP1+CAFs were upregulated, as well as the genes and signaling pathways associated with tumor migration and invasion, and in addition, there was a significant increase in F6_MMP1+CAFs in the tumor tissue of CRC patients with liver metastasis (P<0.000 1). As a ligand, NRG1 expressed by F6_MMP1+CAFs interacted with ERBB3 receptor expressed by tumor cells, and the in vitro experiments confirmed that NRG1 promoted the migration and invasion abilities of tumor cells by activating the ERBB signaling pathway (P<0.05). Conclusion F6_MMP1+CAFs may affect the efficacy of PD-1 immunotherapy in CRC patients and play an important role in promoting liver metastasis in CRC. F6_MMP1+CAFs, along with NRG1 that is produced by them and can promote tumor metastasis, can be used as potential therapeutic targets and prognostic markers for CRC. -
Key words:
- Colorectal Neoplasms /
- Neoplasm Metastasis /
- Therapeutics
-
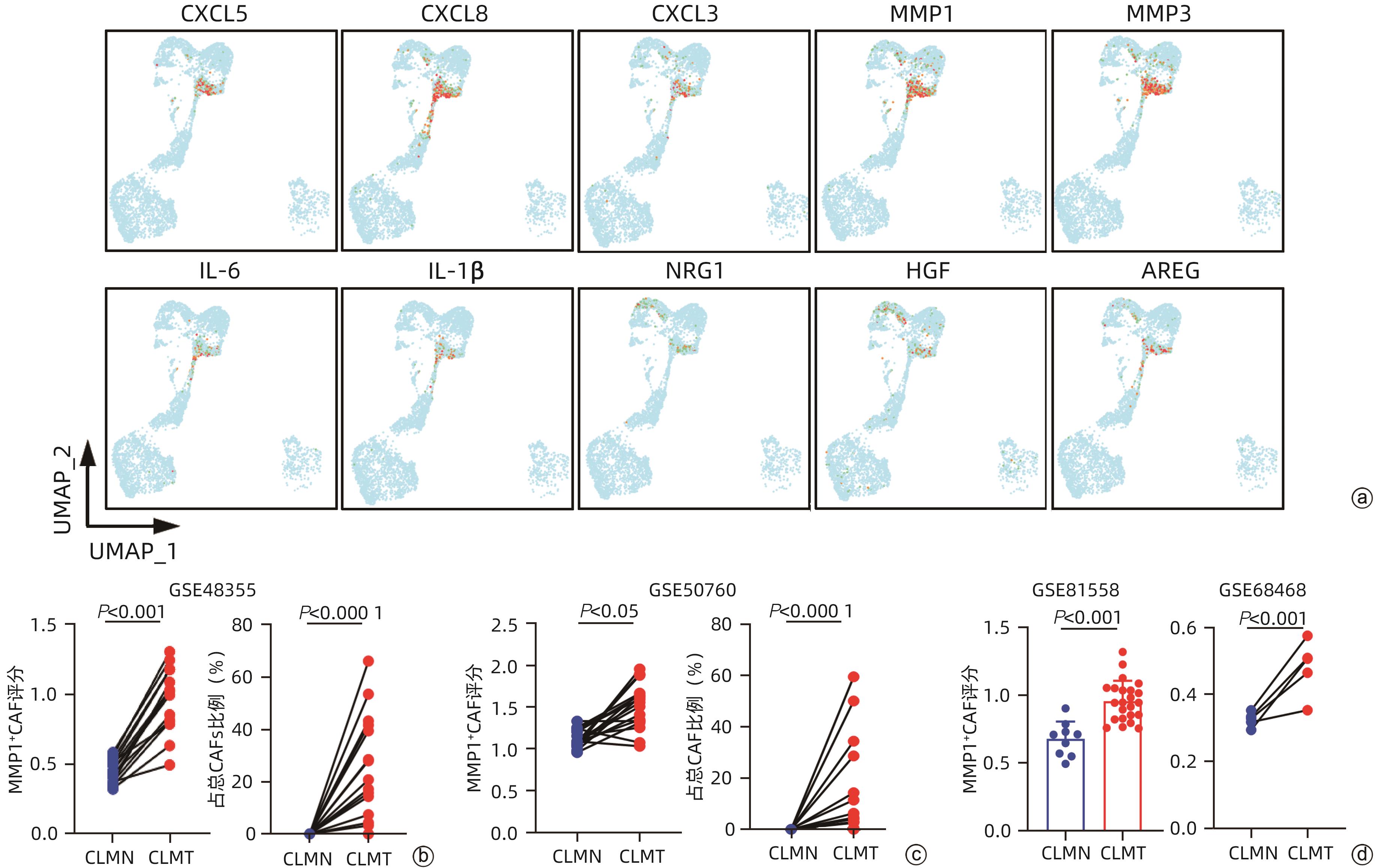
注: a,F6_MMP1高表达基因展示图;b,RNA测序数据库GSE49355中CRC肝转移患者肠道正常组织和原发灶肿瘤组织中F6_MMP1评分和比例配对统计,CLMN:CRC肝转移患者肠道正常组织,CLMT:CRC肝转移患者肠道肿瘤组织;c,RNA测序数据库GSE50760中CRC肝转移患者正常组织和原发灶肿瘤组织中F6_MMP1评分和比例配对统计;d,RNA测序数据库GSE81558和GSE68468中CRC肝转移患者正常组织和原发灶肿瘤组织中F6_MMP1评分统计。
图 3 F6_MMP1+CAF可能与CRC发生肝转移过程密切相关
Figure 3. F6_MMP1+CAFs may be related to the liver metastasis of CRC
-
[1] DEKKER E, TANIS PJ, VLEUGELS JLA, et al. Colorectal cancer[J]. Lancet, 2019, 394( 10207): 1467- 1480. DOI: 10.1016/S0140-6736(19)32319-0. [2] HUANG XY, SHI GM, ZHOU J. Opportunities and challenges for the treatment of malignant hepatobiliary tumors in the new era of immunotherapy[J]. J Clin Hepatol, 2022, 38( 5): 977- 979. DOI: 10.3969/j.issn.1001-5256.2022.05.001.黄晓勇, 施国明, 周俭. 免疫治疗新时代下肝胆恶性肿瘤治疗的机遇和挑战[J]. 临床肝胆病杂志, 2022, 38( 5): 977- 979. DOI: 10.3969/j.issn.1001-5256.2022.05.001. [3] LE DT, URAM JN, WANG H, et al. PD-1 blockade in tumors with mismatch-repair deficiency[J]. N Engl J Med, 2015, 372( 26): 2509- 2520. DOI: 10.1056/NEJMoa1500596. [4] ZHOU H, LIU ZT, WANG YX, et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy[J]. Signal Transduct Target Ther, 2022, 7( 1): 70. DOI: 10.1038/s41392-022-00922-2. [5] MAO XQ, XU J, WANG W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives[J]. Mol Cancer, 2021, 20( 1): 131. DOI: 10.1186/s12943-021-01428-1. [6] LI C, TEIXEIRA AF, ZHU HJ, et al. Cancer associated-fibroblast-derived exosomes in cancer progression[J]. Mol Cancer, 2021, 20( 1): 154. DOI: 10.1186/s12943-021-01463-y. [7] MANTOVANI A, MARCHESI F, JAILLON S, et al. Tumor-associated myeloid cells: Diversity and therapeutic targeting[J]. Cell Mol Immunol, 2021, 18( 3): 566- 578. DOI: 10.1038/s41423-020-00613-4. [8] TANAKA R, KIMURA K, EGUCHI S, et al. Interleukin-8 produced from cancer-associated fibroblasts suppresses proliferation of the OCUCh-LM1 cancer cell line[J]. BMC Cancer, 2022, 22( 1): 748. DOI: 10.1186/s12885-022-09847-z. [9] BARRETT T, WILHITE SE, LEDOUX P, et al. NCBI GEO: Archive for functional genomics data sets: Update[J]. Nucleic Acids Res, 2013, 41( Database issue): D991- D995. DOI: 10.1093/nar/gks1193. [10] LI JX, WU C, HU HB, et al. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer[J]. Cancer Cell, 2023, 41( 6): 1152- 1169. e 7. DOI: 10.1016/j.ccell.2023.04.011. [11] FOLEY CJ, LUO C, O'CALLAGHAN K, et al. Matrix metalloprotease-1a promotes tumorigenesis and metastasis[J]. J Biol Chem, 2012, 287( 29): 24330- 24338. DOI: 10.1074/jbc.M112.356303. [12] WAN XY, GUAN SD, HOU YX, et al. FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts[J]. Theranostics, 2021, 11( 10): 4975- 4991. DOI: 10.7150/thno.55074. [13] MA ZK, LI XD, MAO YZ, et al. Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer[J]. Cancer Cell, 2022, 40( 12): 1550- 1565. e 7. DOI: 10.1016/j.ccell.2022.11.005. [14] KUMAR V, RAMNARAYANAN K, SUNDAR R, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer[J]. Cancer Discov, 2022, 12( 3): 670- 691. DOI: 10.1158/2159-8290.CD-21-0683. [15] DONATO C, KUNZ L, CASTRO-GINER F, et al. Hypoxia triggers the intravasation of clustered circulating tumor cells[J]. Cell Rep, 2020, 32( 10): 108105. DOI: 10.1016/j.celrep.2020.108105. [16] LEQUEUX A, NOMAN MZ, XIAO M, et al. Targeting HIF-1 alpha transcriptional activity drives cytotoxic immune effector cells into melanoma and improves combination immunotherapy[J]. Oncogene, 2021, 40( 28): 4725- 4735. DOI: 10.1038/s41388-021-01846-x. [17] ZHONG BP, CHENG B, HUANG XM, et al. Colorectal cancer-associated fibroblasts promote metastasis by up-regulating LRG1 through stromal IL-6/STAT3 signaling[J]. Cell Death Dis, 2021, 13( 1): 16. DOI: 10.1038/s41419-021-04461-6. [18] KALLURI R. The biology and function of fibroblasts in cancer[J]. Nat Rev Cancer, 2016, 16( 9): 582- 598. DOI: 10.1038/nrc.2016.73. [19] LI XQ, XU K. Mechanism of cancer-associated fibroblasts promoting tumor metastasis and invasion[J]. Chin J Biochem Mol Biol, 2019, 35( 4): 386- 392. DOI: 10.13865/j.cnki.cjbmb.2019.04.06.李学勤, 徐克. 肿瘤相关成纤维细胞促进肿瘤侵袭转移的作用机制[J]. 中国生物化学与分子生物学报, 2019, 35( 4): 386- 392. DOI: 10.13865/j.cnki.cjbmb.2019.04.06. [20] ZHANG W, WANG HS, SUN MY, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target[J]. Cancer Commun, 2020, 40( 2-3): 69- 80. DOI: 10.1002/cac2.12010. [21] ZHOU SL, DAI Z, ZHOU ZJ, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma[J]. Hepatology, 2012, 56( 6): 2242- 2254. DOI: 10.1002/hep.25907. [22] ZHAO JK, OU BC, HAN DP, et al. Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways[J]. Mol Cancer, 2017, 16( 1): 70. DOI: 10.1186/s12943-017-0629-4. [23] SUN XT, HE XK, ZHANG Y, et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism[J]. Gut, 2022, 71( 1): 129- 147. DOI: 10.1136/gutjnl-2020-322744. [24] XIONG XY, LIAO XY, QIU S, et al. CXCL8 in tumor biology and its implications for clinical translation[J]. Front Mol Biosci, 2022, 9: 723846. DOI: 10.3389/fmolb.2022.723846. [25] JONES SA, JENKINS BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer[J]. Nat Rev Immunol, 2018, 18( 12): 773- 789. DOI: 10.1038/s41577-018-0066-7. [26] MCANDREWS KM, CHEN Y, DARPOLOR JK, et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer[J]. Cancer Discov, 2022, 12( 6): 1580- 1597. DOI: 10.1158/2159-8290.CD-20-1484. [27] HAN Y, ZHANG YY, PAN YQ, et al. IL-1β-associated NNT acetylation orchestrates iron-sulfur cluster maintenance and cancer immunotherapy resistance[J]. Mol Cell, 2023, 83( 11): 1887- 1902. e 8. DOI: 10.1016/j.molcel.2023.05.011. [28] ZHANG ZD, KARTHAUS WR, LEE YS, et al. Tumor microenvironment-derived NRG1 promotes antiandrogen resistance in prostate cancer[J]. Cancer Cell, 2020, 38( 2): 279- 296. e 9. DOI: 10.1016/j.ccell.2020.06.005. [29] WEI DY, GENG F, LIANG SM, et al. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells[J]. Biosci Rep, 2017, 37( 2): BSR20160470. DOI: 10.1042/BSR20160470. [30] XU QX, CHIAO P, SUN Y. Amphiregulin in cancer: New insights for translational medicine[J]. Trends Cancer, 2016, 2( 3): 111- 113. DOI: 10.1016/j.trecan.2016.02.002. [31] EREZ N, TRUITT M, OLSON P, et al. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner[J]. Cancer Cell, 2010, 17( 2): 135- 147. DOI: 10.1016/j.ccr.2009.12.041. [32] QI JJ, SUN HX, ZHANG Y, et al. Single-cell and spatial analysis reveal interaction of FAP+ fibroblasts and SPP1+ macrophages in colorectal cancer[J]. Nat Commun, 2022, 13( 1): 1742. DOI: 10.1038/s41467-022-29366-6. -



 PDF下载 ( 4057 KB)
PDF下载 ( 4057 KB)

 下载:
下载: