CC亚族趋化因子及其受体在慢性肝病中的作用及机制
DOI: 10.12449/JCH240528
-
摘要: 近年来,慢性肝病的发病率持续上升,如慢性乙型肝炎、非酒精性脂肪性肝病、肝纤维化、肝硬化、肝细胞癌等,且发病年龄逐渐呈现低龄化趋势。目前许多CC亚族趋化因子在慢性肝病中的作用得到了证实,本文对近年来影响慢性肝病的CC亚家族趋化因子及其受体的研究进展进行总结,探讨其在慢性肝病中的应用潜力,以期为慢性肝病的防治研究提供新思路。Abstract: In recent years, the incidence rate of chronic liver diseases continues to rise, such as chronic hepatitis B, nonalcoholic fatty liver disease, liver fibrosis, liver cirrhosis, and hepatocellular carcinoma, and the age of onset gradually becomes younger. At present, the role of many CC chemokines in chronic liver diseases has been confirmed. This article summarizes the research advances in CC chemokines and their receptors that affect chronic liver diseases in recent years and explore their potential application in chronic liver diseases, so as to provide new ideas for the prevention and treatment of chronic liver diseases.
-
Key words:
- Liver Diseases /
- Chemotactic Factors /
- Receptors, Chemokine
-
表 1 CC亚族趋化因子及其受体在慢性肝病中的作用
Table 1. Role of CC subfamily chemokines and receptors in chronic liver disease
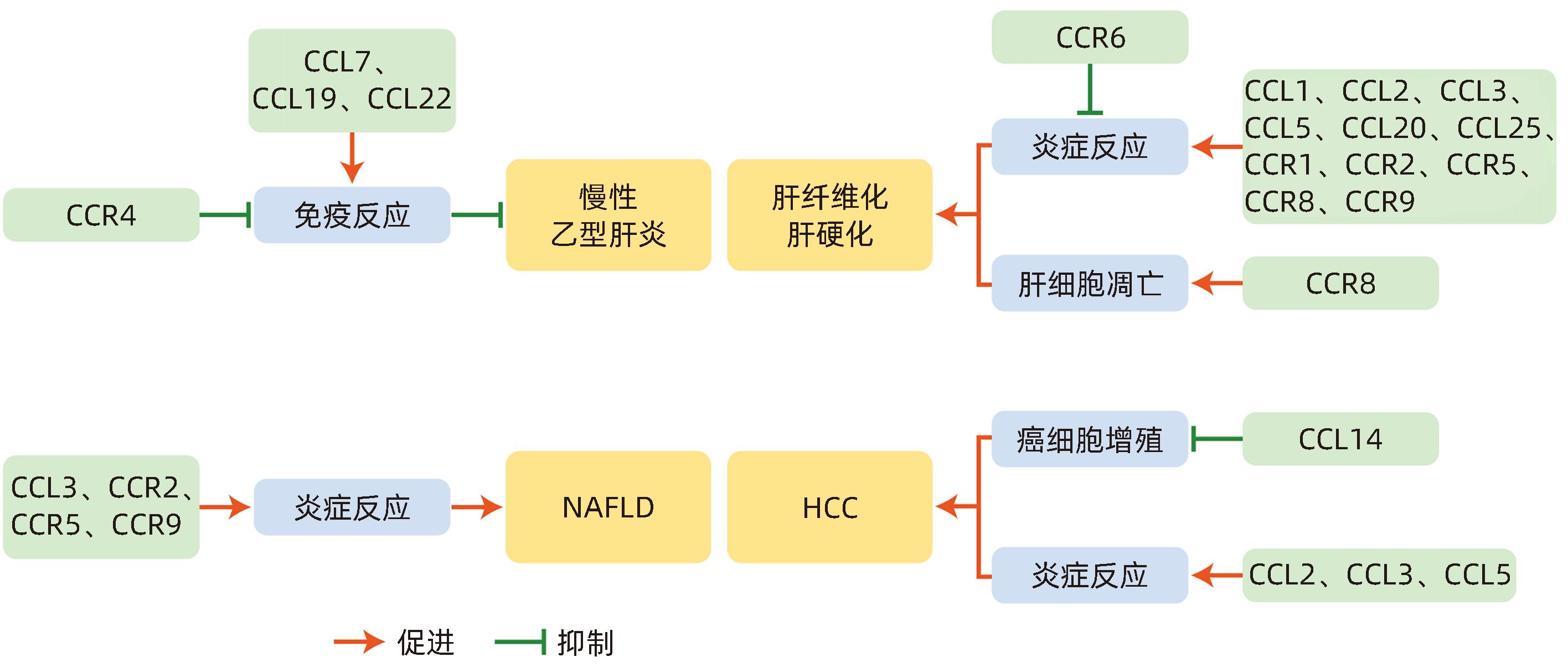
趋化因子 趋化因子受体 在慢性肝病中的作用 CCL1 CCR8 CCR8促进炎性单核细胞向损伤肝脏的迁移,并使其分化为具有促炎表型的巨噬细胞,发挥促纤维化作用 CCL2 CCR2 CCL2与其受体CCR2结合招募单核-巨噬细胞到损伤的肝脏,抑制CCL2的表达,可减少肝纤维化- HCC动物模型中的Ⅰ型胶原、Ⅳ型胶原和病理性血管生成,从而改善肝脏结构紊乱,高浓度CCL2及其受体可对单核/巨噬细胞积聚、炎症过程启动以及纤维蛋白生成产生影响,并促进癌症的发生 CCL3/CCL5 CCR1/CCR5 CCL3表达促进了HSC的增殖和迁移、促进巨噬细胞浸润到肝脏,分化为M1表型,CCL3缺乏可减轻小鼠肝纤维化。CCL3可以向HCC募集白细胞,促进HCC的发生。CCL3是NAFLD进展的致病因素。CCL5可通过ROS的产生、Akt/ERK的信号传导,诱导HSC迁移;并在纤维化和伤口愈合过程中,招募CCR5阳性的肝祖细胞。CCR5和CCR1在慢性肝损伤的情况下可以促进肝纤维化。CVC是CCR2/CCR5双重拮抗剂,通过抑制炎症性FSCN1+巨噬细胞和HERC6+中性粒细胞的肝脏积聚来改善细胞外基质沉积和肝纤维化。CCL5为HCC诊断的潜在生物标志物,CCL5的灵敏度和特异度较CCL4更高 CCL4 CCL4为HCC诊断的潜在生物标志物 CCL11 CCL11通过刺激Jagged 1的转录以调节HSC的活化,发挥促纤维化的作用 CCL14 CCL14为肿瘤抑制因子,可用于判断HCC患者的预后 CCL15 CCL15的高表达与HCC患者的不良预后相关 CCL16 CCL16通过使HSC失活抑制肝硬化的进展,并可作为肝硬化发生、进展的标志物 CCL17 CCR4 CCL17可募集Th17细胞、细胞毒性T淋巴细胞到慢性乙型肝炎患者的肝组织中,阻断CCR4可重建T淋巴细胞抗病毒的免疫应答,并限制Treg的免疫抑制功能,有望成为慢性乙型肝炎的潜在治疗靶点 CCL19 过表达CCL19,可通过增加肝内CD8+T淋巴细胞,快速清除肝内HBV CCL20 CCR6 CCL20可招募巨噬细胞和HSC,并通过激活HSC,发挥促炎及促肝纤维化的作用 CCL25 CCR9 在NASH小鼠巨噬细胞招募和肝纤维化形成中发挥重要作用。CCR9拮抗剂可阻碍肝纤维化、脂肪性肝炎和HCC进展 -
[1] GRIFFITH JW, SOKOL CL, LUSTER AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity[J]. Annu Rev Immunol, 2014, 32: 659- 702. DOI: 10.1146/annurev-immunol-032713-120145. [2] EDDERKAOUI B. Potential role of chemokines in fracture repair[J]. Front Endocrinol(Lausanne), 2017, 8: 39. DOI: 10.3389/fendo.2017.00039. [3] FAHEY S, DEMPSEY E, LONG A. The role of chemokines in acute and chronic hepatitis C infection[J]. Cell Mol Immunol, 2014, 11( 1): 25- 40. DOI: 10.1038/cmi.2013.37. [4] WASMUTH HE, TACKE F, TRAUTWEIN C. Chemokines in liver inflammation and fibrosis[J]. Semin Liver Dis, 2010, 30( 3): 215- 225. DOI: 10.1055/s-0030-1255351. [5] GUSTAVSSON M. New insights into the structure and function of chemokine receptor: Chemokine complexes from an experimental perspective[J]. J Leukoc Biol, 2020, 107( 6): 1115- 1122. DOI: 10.1002/JLB.2MR1219-288R. [6] CHARO IF, RANSOHOFF RM. The many roles of chemokines and chemokine receptors in inflammation[J]. N Engl J Med, 2006, 354( 6): 610- 621. DOI: 10.1056/NEJMra052723. [7] BAGGIOLINI M, LOETSCHER P. Chemokines in inflammation and immunity[J]. Immunol Today, 2000, 21( 9): 418- 420. DOI: 10.1016/s0167-5699(00)01672-8. [8] SALLUSTO F, MACKAY CR, LANZAVECCHIA A. The role of chemokine receptors in primary, effector, and memory immune responses[J]. Annu Rev Immunol, 2000, 18: 593- 620. DOI: 10.1146/annurev.immunol.18.1.593. [9] WHITE GE, IQBAL AJ, GREAVES DR. CC chemokine receptors and chronic inflammation: Therapeutic opportunities and pharmacological challenges[J]. Pharmacol Rev, 2013, 65( 1): 47- 89. DOI: 10.1124/pr.111.005074. [10] SEEGER C, MASON WS. Molecular biology of hepatitis B virus infection[J]. Virology, 2015, 479-480: 672- 686. DOI: 10.1016/j.virol.2015.02.031. [11] HU LS, ZHU Y, ZHANG JQ, et al. Potential circulating biomarkers of circulating chemokines CCL5, MIP-1β and HA as for early detection of cirrhosis related to chronic HBV(hepatitis B virus) infection[J]. BMC Infect Dis, 2019, 19( 1): 523. DOI: 10.1186/s12879-019-4130-0. [12] LI Y, WANG CM, ZHAO T, et al. Hepatitis B virus X protein modulates chemokine CCL15 upregulation in hepatocellular carcinoma[J]. Anticancer Agents Med Chem, 2021, 21( 16): 2198- 2203. DOI: 10.2174/1871520621666210302083407. [13] ZHANG K, LIU YQ, YANG XA, et al. HBV promotes the recruitment of IL-17 secreting T cells via chemokines CCL22 and CCL17[J]. Liver Int, 2020, 40( 6): 1327- 1338. DOI: 10.1111/liv.14438. [14] ZHUO JY, LU D, LIN ZY, et al. CC motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells[J]. Hepatobiliary Pancreat Dis Int, 2020, 19( 5): 440- 448. DOI: 10.1016/j.hbpd.2019.12.006. [15] YAN Y, ZHAO W, LIU W, et al. CCL19 enhances CD8+ T-cell responses and accelerates HBV clearance[J]. J Gastroenterol, 2021, 56( 8): 769- 785. DOI: 10.1007/s00535-021-01799-8. [16] KHANAM A, GHOSH A, CHUA JV, et al. Blockade of CCR4 breaks immune tolerance in chronic hepatitis B patients by modulating regulatory pathways[J]. J Transl Med, 2023, 21( 1): 271. DOI: 10.1186/s12967-023-04104-8. [17] LIU HJ, GUAN QZ, ZHAO P, et al. TGF-β-induced CCR8 promoted macrophage transdifferentiation into myofibroblast-like cells[J]. Exp Lung Res, 2022: 1- 14. DOI: 10.1080/01902148.2022.2055227. [18] LI L, WEI W, LI ZZ, et al. The spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis[J]. Cell Physiol Biochem, 2018, 51( 2): 557- 574. DOI: 10.1159/000495276. [19] BARTNECK M, SCHRAMMEN PL, MÖCKEL D, et al. The CCR2+ macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers[J]. Cell Mol Gastroenterol Hepatol, 2019, 7( 2): 371- 390. DOI: 10.1016/j.jcmgh.2018.10.007. [20] FLAMINI S, SERGEEV P, VIANA de BARROS Z, et al. Glucocorticoid-induced leucine zipper regulates liver fibrosis by suppressing CCL2-mediated leukocyte recruitment[J]. Cell Death Dis, 2021, 12( 5): 421. DOI: 10.1038/s41419-021-03704-w. [21] XI S, ZHENG X, LI X, et al. Activated hepatic stellate cells induce infiltration and formation of CD163+ macrophages via CCL2/CCR2 pathway[J]. Front Med(Lausanne). 2021, 8: 627927. DOI: 10.3389/fmed.2021.627927. [22] DU PLESSIS J, KORF H, van PELT J, et al. Pro-inflammatory cytokines but not endotoxin-related parameters associate with disease severity in patients with NAFLD[J]. PLoS One, 2016, 11( 12): e0166048. DOI: 10.1371/journal.pone.0166048. [23] XU L, CHEN YP, NAGASHIMADA M, et al. CC chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice[J]. Metabolism, 2021, 125: 154914. DOI: 10.1016/j.metabol.2021.154914. [24] GUO YK, ZHAO C, DAI WT, et al. C-C motif chemokine receptor 2 inhibition reduces liver fibrosis by restoring the immune cell landscape[J]. Int J Biol Sci, 2023, 19( 8): 2572- 2587. DOI: 10.7150/ijbs.83530. [25] IGAKI K, KOMOIKE Y, NAKAMURA Y, et al. MLN3126, an antagonist of the chemokine receptor CCR9, ameliorates inflammation in a T cell mediated mouse colitis model[J]. Int Immunopharmacol, 2018, 60: 160- 169. DOI: 10.1016/j.intimp.2018.04.049. [26] MORIKAWA R, NAKAMOTO N, AMIYA T, et al. Role of CC chemokine receptor 9 in the progression of murine and human non-alcoholic steatohepatitis[J]. J Hepatol, 2021, 74( 3): 511- 521. DOI: 10.1016/j.jhep.2020.09.033. [27] HANSON A, PIRAS IS, WILHELMSEN D, et al. Chemokine ligand 20(CCL20) expression increases with NAFLD stage and hepatic stellate cell activation and is regulated by miR-590-5p[J]. Cytokine, 2019, 123: 154789. DOI: 10.1016/j.cyto.2019.154789. [28] HEO YJ, CHOI SE, LEE NM, et al. CCL20 induced by visfatin in macrophages via the NF-κB and MKK3/6-p38 signaling pathways contributes to hepatic stellate cell activation[J]. Mol Biol Rep, 2020, 47( 6): 4285- 4293. DOI: 10.1007/s11033-020-05510-7. [29] KISSELEVA T, BRENNER D. Molecular and cellular mechanisms of liver fibrosis and its regression[J]. Nat Rev Gastroenterol Hepatol, 2021, 18( 3): 151- 166. DOI: 10.1038/s41575-020-00372-7. [30] KONG M, DONG WH, KANG AQ, et al. Regulatory role and translational potential of CCL11 in liver fibrosis[J]. Hepatology, 2023, 78( 1): 120- 135. DOI: 10.1097/HEP.0000000000000287. [31] PROOST P, WUYTS A, van DAMME J. The role of chemokines in inflammation[J]. Int J Clin Lab Res, 1996, 26( 4): 211- 223. DOI: 10.1007/BF02602952. [32] TACKE F. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo[J]. Fibrogenesis Tissue Repair, 2012, 5( Suppl 1): S27. DOI: 10.1186/1755-1536-5-S1-S27. [33] DEBES JD, ROMAGNOLI PA, PRIETO J, et al. Serum biomarkers for the prediction of hepatocellular carcinoma[J]. Cancers(Basel), 2021, 13( 7): 1681. DOI: 10.3390/cancers13071681. [34] ZHU MX, XU WY, WEI CY, et al. CCL14 serves as a novel prognostic factor and tumor suppressor of HCC by modulating cell cycle and promoting apoptosis[J]. Cell Death Dis, 2019, 10( 11): 796. DOI: 10.1038/s41419-019-1966-6. [35] EHLING J, TACKE F. Role of chemokine pathways in hepatobiliary cancer[J]. Cancer Lett, 2016, 379( 2): 173- 183. DOI: 10.1016/j.canlet.2015.06.017. [36] YANG XQ, LU PR, FUJII C, et al. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression[J]. Int J Cancer, 2006, 118( 8): 1869- 1876. DOI: 10.1002/ijc.21596. [37] PAN XF, CHIWANDA KAMINGA A, LIU AZ, et al. Chemokines in non-alcoholic fatty liver disease: A systematic review and network meta-analysis[J]. Front Immunol, 2020, 11: 1802. DOI: 10.3389/fimmu.2020.01802. [38] TAN YY, YUE SR, LU AP, et al. The improvement of nonalcoholic steatohepatitis by Poria cocos polysaccharides associated with gut microbiota and NF-κB/CCL3/CCR1 axis[J]. Phytomedicine, 2022, 103: 154208. DOI: 10.1016/j.phymed.2022.154208. [39] RATZIU V, SANYAL A, HARRISON SA, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: Final analysis of the phase 2b CENTAUR study[J]. Hepatology, 2020, 72( 3): 892- 905. DOI: 10.1002/hep.31108. [40] WIERING L, TACKE F. Treating inflammation to combat non-alcoholic fatty liver disease[J]. J Endocrinol, 2022, 256( 1): e220194. DOI: 10.1530/JOE-22-0194. [41] PUENGEL T, LEFERE S, HUNDERTMARK J, et al. Combined therapy with a CCR2/CCR5 antagonist and FGF21 analogue synergizes in ameliorating steatohepatitis and fibrosis[J]. Int J Mol Sci, 2022, 23( 12): 6696. DOI: 10.3390/ijms23126696. -



 PDF下载 ( 754 KB)
PDF下载 ( 754 KB)

 下载:
下载:


