妊娠期急性胰腺炎的临床特征及危险因素分析
DOI: 10.12449/JCH240522
-
摘要:
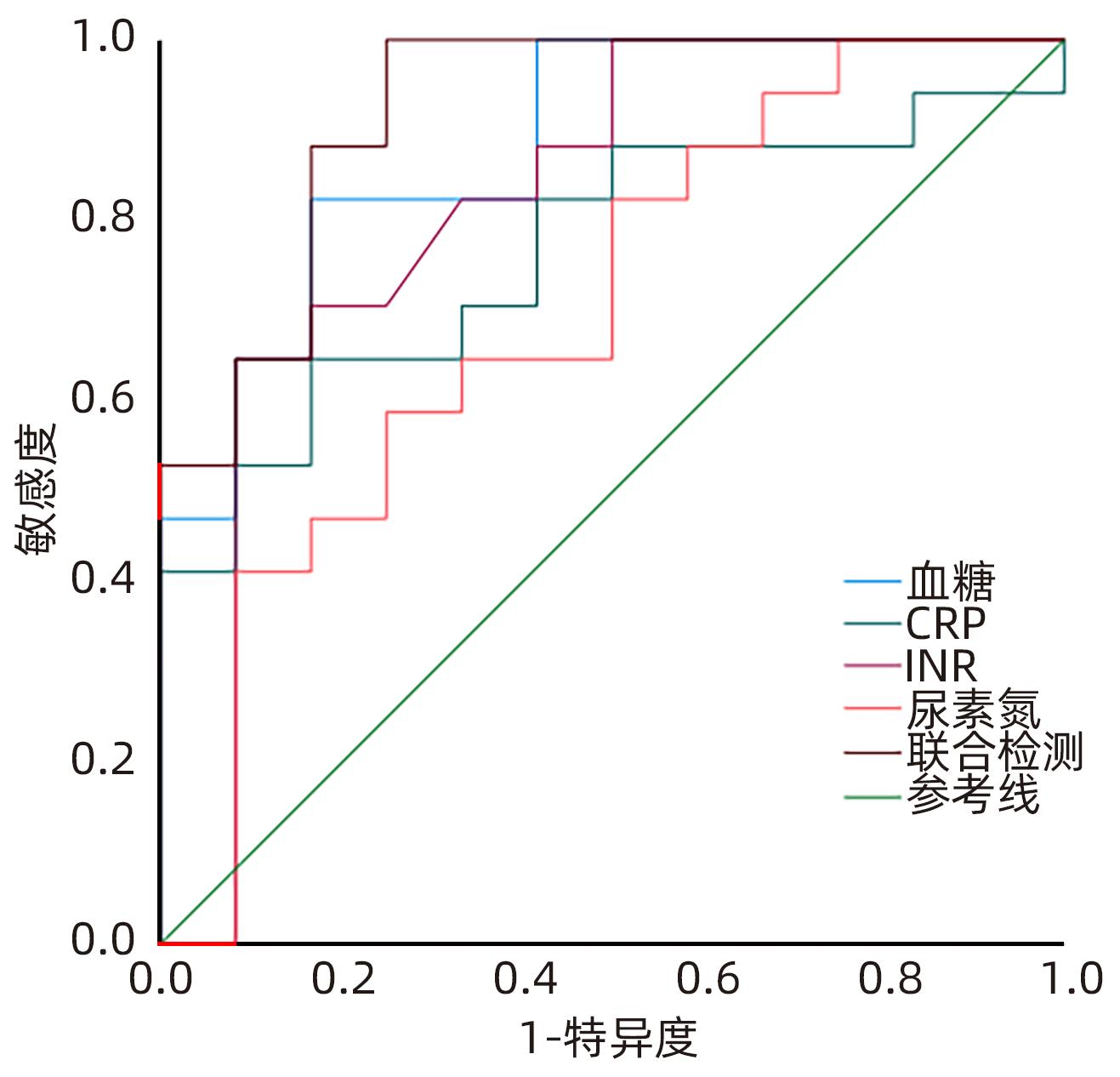
目的 分析妊娠期急性胰腺炎(APIP)的临床特征及母婴结局,探讨病情加重的危险因素并试图建立预测模型。 方法 选取2017年1月—2022年12月遵义医科大学附属医院收治的52例APIP患者,按病情严重程度分为轻型急性胰腺炎(MAP,n=32)、中度重症胰腺炎(MSAP,n=8)和重症胰腺炎(SAP,n=12),并进行回顾性分析。对各组临床资料进行Logistic回归分析,绘制受试者工作特征曲线(ROC曲线)评估危险因素对APIP病情严重程度的预测价值。符合正态分布计量资料多组间比较采用单因素方差分析,进一步两两比较采用LSD-t检验;非正态分布的计量资料多组间比较采用Kruskal-Wallis H检验,进一步两两比较采用Wilcoxon检验。计数资料组间比较采用χ2检验。 结果 52例APIP患者按病因分组:26例(50.0%)患者为高脂性胰腺炎、20例(38.5%)患者为胆源性胰腺炎、6例(11.5%)患者为特发性胰腺炎。依据孕周分组:1例(1.9%)患者处在妊娠早期、25例(48.1%)患者处在妊娠中期、26例(50.0%)患者处在妊娠晚期。10例(19.2%)患者并发急性呼吸窘迫综合征(ARDS),其中9例(90%)使用了呼吸机支持。不同严重程度APIP患者间AST、ALT、尿素氮、血糖、CRP、INR、是否肺炎、是否ARDS、是否脓毒症、是否肝功能不全和是否凝血功能不全的组间差异均有统计学意义(P值均<0.05)。单因素分析显示,APIP严重程度与血糖、尿素氮、CRP和肺炎有相关性(P值均<0.05),肺炎是APIP病情加重的危险因素(OR=18.938,95%CI:1.020~351.747,P=0.048)。CRP、血糖、尿素氮、INR联合预测APIP严重程度的ROC曲线下面积(0.954)高于CRP、血糖、尿素氮、INR的单独检测值(0.778、0.796、0.721、0.801)。 结论 肺炎是APIP病情加重的危险因素,CRP、血糖、尿素氮、INR可联合预测APIP严重程度。 Abstract:Objective To investigate the clinical features and maternal and fetal outcomes of acute pancreatitis in pregnancy (APIP) and the risk factors for disease aggravation, and to establish a predictive model. Methods A retrospective analysis was performed for 52 APIP patients who were admitted to Affiliated Hospital of Zunyi Medical University from January 2017 to December 2022, and according to disease severity, they were divided into mild acute pancreatitis (MAP) group with 32 patients, moderate-severe acute pancreatitis (MSAP) group with 8 patients, and severe acute pancreatitis (SAP) group with 12 patients. The logistic regression analysis was performed for the clinical data of each group,and the receiver operating characteristic (ROC) curves were plotted to assess the value of risk factors in predicting the severity of APIP. A one-way analysis of variance was used for comparison of normally distributed continuous data between multiple groups,and the least significant difference t-test was used for further comparision between two groups. The Kruskal-Wallis H test was used for comparison of non-normally distributed continuous data between multiple groups,and the Wilcoxon rank-sum test was used for further comparision between two groups; the chi-square test was used for comparison of categorical data between groups. Results Of all patients in terms of etiology, 26 (50%) had hyperlipidemic pancreatitis, 20 (38.4%) had biliary pancreatitis, and 6 (11.5%) had idiopathic pancreatitis. In terms of gestational week, 1 patient (1.9%) was in early pregnancy, 25 (48.1%) were in mid-pregnancy, and 26 (50.0%) were in late pregnancy. A total of 10 patients (19.2%) had acute respiratory distress syndrome (ARDS), among whom 9 (90%) required respiratory support. There were significant differences between the patients with different severities of APIP in aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, blood glucose, C-reactive protein (CRP), international normalized ratio (INR), pneumonia, ARDS, sepsis, hepatic insufficiency, and coagulation dysfunction (all P<0.05). The univariate analysis showed that the severity of APIP was associated with blood glucose, blood urea nitrogen, CRP, and pneumonia (all P<0.05), and pneumonia was a risk factor for the aggravation of APIP (odds ratio=18.938, 95% confidence interval: 1.020 — 351.747, P=0.048). CRP, blood glucose, blood urea nitrogen, and INR used in combination had a larger area under the ROC curve than each index used alone (0.954 vs 0.778/0.796/0.721/0.801). Conclusion Pneumonia is a risk factor for the aggravation of APIP, and the combination of CRP, blood glucose, blood urea nitrogen, and INR can be used to predict the severity of APIP. -
Key words:
- Pancreatitis /
- Pregnant Women /
- Prognosis /
- Risk Factors
-
表 1 不同严重程度APIP患者的基线特征
Table 1. Baseline characteristics of APIP patients with different severity
项目 MAP(n=32) MSAP(n=8) SAP(n=12) 统计值 P值 年龄(岁) 29.00±5.84 27.00±5.95 31.08±4.80 F=1.299 0.282 初产妇[例(%)] 23(71.8) 4(50.0) 8(66.7) χ2=1.507 0.534 发病孕期[例(%)] χ2=1.783 0.434 早期 0(0.0) 0(0.0) 1(8.3) 中期 18(56.3) 3(37.5) 4(33.3) 晚期 14(43.8) 5(62.5) 7(58.3) 病因[例(%)] 胆源性 13(40.6) 5(62.5) 2(16.7) χ2=8.159 0.059 高脂性 16(50.0) 1(12.5) 9(75.0) 特发性 3(9.4) 2(25.0) 1(8.3) 水肿型[例(%)] 27(84.4)1) 7(87.5)1) 3(25.0) χ2=14.302 0.001 入住ICU[例(%)] 3(9.4)1) 5(62.5)1) 12(100.0) χ2=34.993 <0.001 ICU停留时间(天) 0.00(0.00~0.00)1) 3.00(0.00~5.00)1) 9.50(7.00~11.75) H=7.955 0.019 住院时间(天) 5.00(3.25~6.75) 14.00(8.00~17.75)2) 14.50(12.25~45.25)2) H=23.095 <0.001 治疗费用(元) 3 988.34 (1 573.14~10 175.92)1) 44 933.84 (9 547.35~71 369.44)2) 75 828.25 (48 981.25~192 749.73) H=28.702 <0.001 注:与SAP组比较,1)P<0.05;与MAP组比较,2)P<0.05。 表 2 不同严重程度APIP患者的生化指标比较
Table 2. Comparison of biochemical indices in patients with APIP of different severities
指标 MAP(n=32) MSAP(n=8) SAP(n=12) 统计值 P值 ALT(U/L) 18.00(9.50~25.50) 69.00(16.25~131.75)1) 10.50(5.50~25.00) H=7.102 0.029 AST(U/L) 30.00(21.00~40.00) 79.00(34.00~143.25) 1) 26.00(17.75~66.50) H=6.366 0.041 尿素氮(mmol/L) 2.53(1.93~3.49) 1) 3.75(2.43~6.26) 3.55(2.56~6.39) H=7.077 0.029 血糖(mmol/L) 4.25(3.42~5.29) 1) 5.54(5.10~6.94) 7.01(5.67~7.88) H=9.935 0.007 CRP(mg/L) 35.74(3.38~128.45) 1) 127.72(29.99~173.53) 154.20(59.22~175.22) H=7.288 0.026 INR 0.84(0.78~0.92) 1) 0.89(0.83~0.95) 1.00(0.90~1.20) H=10.081 0.006 NLR 9.00(4.00~17.50) 1) 17.50(10.00~24.50) 16.00(8.25~21.00) H=6.236 0.044 血淀粉酶(U/L) 254.00(127.00~960.00) 414.00(142.00~1 040.50) 225.00(77.00~677.00) H=1.036 0.596 尿淀粉酶(U/L) 2 720.00 (1 477.00~7 041.00) 1 285.00 (374.00~3 664.00) 535.00 (397.00~7 336.75) H=2.172 0.338 白细胞计数(×109/L) 12.94(9.03~16.94) 15.00(14.12~16.03) 16.12(10.89~20.37) H=4.140 0.126 白蛋白(g/L) 32.61±4.58 32.88±5.99 32.78±6.32 F=0.056 0.946 肌酐(μmol/L) 47.72±16.06 54.88±16.88 54.83±27.77 F=0.830 0.442 甘油三酯(mmol/L) 12.71(2.50~17.23) 3.32(2.65~4.89) 11.96(4.79~17.48) H=2.469 0.291 PT(s) 14.45±21.17 10.66±0.91 12.70±2.59 F=0.177 0.839 注:与SAP组比较,1)P<0.05。 表 3 不同严重程度APIP患者的并发症及预后比较
Table 3. Complications and prognosis of APIP with different severity
项目 MAP(n=32) MSAP(n=8) SAP(n=12) 统计值 P值 并发症[例(%)] 肺炎 3(9.4)1) 3(37.5) 10(83.3) χ2=21.828 <0.001 肝功能不全 0(0.0) 4(50.0)1)2) 1(8.3) χ2=13.206 0.001 腹腔感染 1(3.1)1) 1(12.5) 5(41.7) χ2=9.529 0.004 凝血功能不全 0(0.0)1) 0(0.0) 2(16.7) χ2=6.933 0.031 ARDS 0(0.0)1) 2(25.0)2) 8(66.7) χ2=23.865 <0.001 脓毒症 0(0.0)1) 0(0.0)1) 6(50.0) χ2=16.810 <0.001 评分 APACHEⅡ评分 9.67±1.531) 12.20±3.901) 14.50±4.40 F=6.068 0.010 Ranson评分 2.0(2.0~2.0) 3.0(2.5~3.5) 3.0(2.5~4.0) H= 4.871 0.088 结局[例(%)] 胎儿存活 29(90.6) 6(75.0) 9(75.0) χ2=2.308 0.315 注:与SAP组比较,1)P<0.05;与MAP组比较,2)P<0.05。 表 4 APIP严重程度危险因素分析
Table 4. Analysis of risk factors of APIP severity
指标 单因素分析 多因素分析 OR P值 95%CI OR P值 95% CI 血糖 1.671 0.014 1.109~2.519 2.825 0.155 0.675~11.820 尿素氮 1.638 0.022 1.075~2.496 1.897 0.205 0.704~5.108 CRP 1.015 0.019 1.002~1.027 1.000 0.975 0.979~1.022 肺炎 48.333 0.001 7.028~332.383 18.938 0.048 1.020~351.747 表 5 不同指标对APIP严重程度预测值
Table 5. Predicted values of APIP severity by different indicators
指标 AUC 敏感度(%) 特异度(%) 准确度(%) P值 CRP 0.778 66.70 83.30 50.00 0.011 血糖 0.796 71.00 83.30 54.30 0.030 尿素氮 0.721 46.90 91.70 38.60 0.025 INR 0.801 67.70 83.30 51.00 0.002 联合检测 0.954 83.30 100.00 83.30 <0.001 -
[1] DATE RS, KAUSHAL M, RAMESH A. A review of the management of gallstone disease and its complications in pregnancy[J]. Am J Surg, 2008, 196( 4): 599- 608. DOI: 10.1016/j.amjsurg.2008.01.015. [2] HUANG CL, LIU J, LU YY, et al. Clinical features and treatment of hypertriglyceridemia-induced acute pancreatitis during pregnancy: A retrospective study[J]. J Clin Apher, 2016, 31( 6): 571- 578. DOI: 10.1002/jca.21453. [3] BANKS PA, BOLLEN TL, DERVENIS C, et al. Classification of acute pancreatitis: 2012: Revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62( 1): 102- 111. DOI: 10.1136/gutjnl-2012-302779. [4] WEI P, ZHAO XL, HOU WH, et al. Clinical characteristics and maternal and fetal outcomes of 166 patients with acute pancreatitis in pregnancy[J]. Chin J Pract Gynecol Obstet, 2022, 38( 7): 756- 759. DOI: 10.19538/j.fk2022070119.魏鹏, 赵先兰, 侯文汇, 等. 妊娠合并急性胰腺炎166例临床特征及母儿结局分析[J]. 中国实用妇科与产科杂志, 2022, 38( 7): 756- 759. DOI: 10.19538/j.fk2022070119. [5] DUCARME G, MAIRE F, CHATEL P, et al. Acute pancreatitis during pregnancy: A review[J]. J Perinatol, 2014, 34( 2): 87- 94. DOI: 10.1038/jp.2013.161. [6] TSUANG W, NAVANEETHAN U, RUIZ L, et al. Hypertriglyceridemic pancreatitis: Presentation and management[J]. Am J Gastroenterol, 2009, 104( 4): 984- 991. DOI: 10.1038/ajg.2009.27. [7] JIANG X, YAN YF, ZHONG R, et al. Clinical features of biliary acute pancreatitis versus hypertriglyceridemic acute pancreatitis[J]. J Clin Hepatol, 2020, 36( 9): 2050- 2055. DOI: 10.3969/j.issn.1001-5256.2020.09.028.蒋鑫, 严永峰, 钟瑞, 等. 胆源性急性胰腺炎与高甘油三酯血症性急性胰腺炎临床特点对比分析[J]. 临床肝胆病杂志, 2020, 36( 9): 2050- 2055. DOI: 10.3969/j.issn.1001-5256.2020.09.028. [8] ONG Y, SHELAT VG. Ranson score to stratify severity in acute pancreatitis remains valid-old is gold[J]. Expert Rev Gastroenterol Hepatol, 2021, 15( 8): 865- 877. DOI: 10.1080/17474124.2021.1924058. [9] Chinese Society for Emergency Medicine, Beijing-Tianjin-Hebei Alliance of Emergency Treatment and First Aid, Emergency Medicine Branch, Beijing Medical Association, et al. Expert consensus on emergency diagnosis and treatment of acute pancreatitis[J]. J Clin Hepatol, 2021, 37( 5): 1034- 1041. DOI: 10.3969/j.issn.1001-5256.2021.05.012.中华医学会急诊分会, 京津冀急诊急救联盟, 北京医学会急诊分会, 等. 急性胰腺炎急诊诊断及治疗专家共识[J]. 临床肝胆病杂志, 2021, 37( 5): 1034- 1041. DOI: 10.3969/j.issn.1001-5256.2021.05.012. [10] PITCHUMONI CS, YEGNESWARAN B. Acute pancreatitis in pregnancy[J]. World J Gastroenterol, 2009, 15( 45): 5641. DOI: 10.3748/wjg.15.5641. [11] GARG PK, SINGH VP. Organ failure due to systemic injury in acute pancreatitis[J]. Gastroenterology, 2019, 156( 7): 2008- 2023. DOI: 10.1053/j.gastro.2018.12.041. [12] SZATMARY P, GRAMMATIKOPOULOS T, CAI WH, et al. Acute pancreatitis: Diagnosis and treatment[J]. Drugs, 2022, 82( 12): 1251- 1276. DOI: 10.1007/s40265-022-01766-4. [13] TANG SJ, RODRIGUEZ-FRIAS E, SINGH S, et al. Acute pancreatitis during pregnancy[J]. Clin Gastroenterol Hepatol, 2010, 8( 1): 85- 90. DOI: 10.1016/j.cgh.2009.08.035. [14] KMN V, SHEELA CN, BANKA S, et al. Maternal and perinatal outcome of acute pancreatitis during pregnancy: A 5 year experience at a tertiary care centre[J]. Int J Reprod Contracept Obstet Gynecol, 2016: 4041- 4045. DOI: 10.18203/2320-1770.ijrcog20163886. [15] GENG YX, LI WQ, SUN LQ, et al. Severe acute pancreatitis during pregnancy: Eleven years experience from a surgical intensive care unit[J]. Dig Dis Sci, 2011, 56( 12): 3672- 3677. DOI: 10.1007/s10620-011-1809-5. [16] PAPADAKIS EP, SARIGIANNI M, MIKHAILIDIS DP, et al. Acute pancreatitis in pregnancy: An overview[J]. Eur J Obstet Gynecol Reprod Biol, 2011, 159( 2): 261- 266. DOI: 10.1016/j.ejogrb.2011.07.037. [17] ZHANG TT, WANG GX, CAO Z, et al. Acute pancreatitis in pregnancy: A 10-year, multi-center, retrospective study in Beijing[J]. BMC Pregnancy Childbirth, 2022, 22( 1): 414. DOI: 10.1186/s12884-022-04742-8. [18] YANG ZY, GUO GY, LI H. Predicting fetal loss in severe acute pancreatitis during pregnancy: A 5-year single-tertiary-center retrospective analysis[J]. Postgrad Med, 2020, 132( 5): 473- 478. DOI: 10.1080/00325481.2020.1752010. [19] RAUSCHERT S, GÁZQUEZ A, UHL O, et al. Phospholipids in lipoproteins: Compositional differences across VLDL, LDL, and HDL in pregnant women[J]. Lipids Health Dis, 2019, 18( 1): 20. DOI: 10.1186/s12944-019-0957-z. [20] RUSSI G. Severe dyslipidemia in pregnancy: The role of therapeutic apheresis[J]. Transfus Apher Sci, 2015, 53( 3): 283- 287. DOI: 10.1016/j.transci.2015.11.008. [21] PENG R, ZHANG XM, ZHANG L, et al. CT findings of acute pancreatitis with pneumonia and its clinical correlation analysis[J]. Radiol Pract, 2020, 35( 1): 68- 73. DOI: 10.13609/j.cnki.1000-0313.2020.01.013.彭容, 张小明, 张凌, 等. 急性胰腺炎伴肺炎的CT表现及其与临床相关性分析[J]. 放射学实践, 2020, 35( 1): 68- 73. DOI: 10.13609/j.cnki.1000-0313.2020.01.013. [22] SHEN HN, CHANG YH, CHEN HF, et al. Increased risk of severe acute pancreatitis in patients with diabetes[J]. Diabet Med, 2012, 29( 11): 1419- 1424. DOI: 10.1111/j.1464-5491.2012.03680.x. [23] URUSHIHARA H, TAKETSUNA M, LIU Y, et al. Increased risk of acute pancreatitis in patients with type 2 diabetes: An observational study using a Japanese hospital database[J]. PLoS One, 2012, 7( 12): e53224. DOI: 10.1371/journal.pone.0053224. [24] SCHÜTTE K, MALFERTHEINER P. Markers for predicting severity and progression of acute pancreatitis[J]. Best Pract Res Clin Gastroenterol, 2008, 22( 1): 75- 90. DOI: 10.1016/j.bpg.2007.10.013. [25] ZHOU HJ, MEI X, HE XH, et al. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: A retrospective study[J]. Medicine(Baltimore), 2019, 98( 16): e15275. DOI: 10.1097/MD.0000000000015275. [26] KONG WH, HE YY, BAO HR, et al. Diagnostic value of neutrophil-lymphocyte ratio for predicting the severity of acute pancreatitis: A meta-analysis[J]. Dis Markers, 2020, 2020: 9731854. DOI: 10.1155/2020/9731854. [27] LIU DL, WEN LL, WANG ZD, et al. The mechanism of lung and intestinal injury in acute pancreatitis: A review[J]. Front Med(Lausanne), 2022, 9: 904078. DOI: 10.3389/fmed.2022.904078. [28] GE JH. Risk factors for development of acute respiratory distress syndrome in septic patients[J]. Zhejiang Med J, 2017, 39( 20): 1789- 1792. DOI: 10.12056/j.issn.1006-2785.2017.39.20.2017-1141.葛建辉. 脓毒症患者并发ARDS的早期危险因素分析[J]. 浙江医学, 2017, 39( 20): 1789- 1792. DOI: 10.12056/j.issn.1006-2785.2017.39.20.2017-1141. [29] CASQUEIRO J, CASQUEIRO J, ALVES C. Infections in patients with diabetes mellitus: A review of pathogenesis[J]. Indian J Endocrinol Metab, 2012, 16( Suppl1): S27- S36. DOI: 10.4103/2230-8210.94253. -



 PDF下载 ( 736 KB)
PDF下载 ( 736 KB)

 下载:
下载:


