急性肝卟啉病的治疗进展
DOI: 10.12449/JCH240430
利益冲突声明:本文不存在任何利益冲突。
作者贡献声明:雷佳佳负责资料搜集与分析,撰写论文;任毅、杨静负责课题设计,修改论文;李霜、董白雪参与资料分析与总结;任毅负责拟定写作思路,指导撰写文章并最后定稿。
-
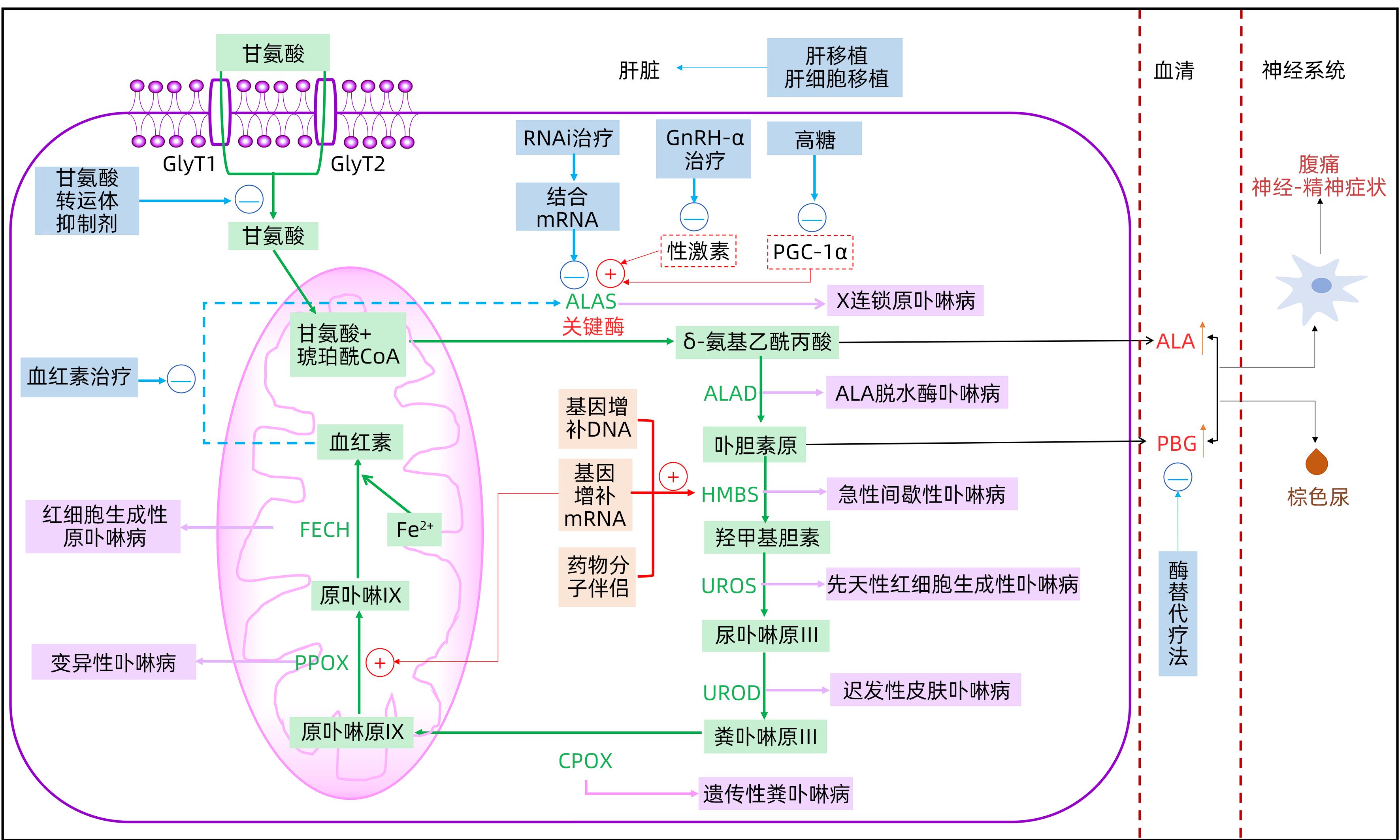
摘要: 急性肝卟啉病(AHP)是一种血红素代谢异常的罕见病,近年来对该病的治疗有了突破。除常规治疗外,本文重点综述了AHP的新疗法,这些治疗正处于初步应用于临床,或仍在研究阶段,包括RNAi疗法、酶替代疗法、DNA或mRNA的基因增补、药物分子伴侣和降低血红素合成的甘氨酸转运体抑制剂等。另外,本文对AHP相关的低钠血症、可逆性后部脑病综合征等合并症、并发症的治疗也进行了综述。我国对于AHP的治疗主要以高糖输注为主,我国诊断水平的提升及对罕见病的关注度增加,促进了AHP的诊治发展,有望今后能够探索更多适宜于我国人群的AHP的治疗方法。Abstract: Acute hepatic porphyria (AHP) is a rare disease with abnormal heme metabolism, and breakthroughs have been made in the treatment of this disease in recent years. In addition to conventional treatment methods, this article reviews new therapies for AHP that are in the stage of initial clinical application or are still in the research stage, including RNAi therapy, enzyme replacement therapy, genetic supplementation of DNA or mRNA, drug molecular chaperones, and glycine transporter inhibitors for reducing heme synthesis. Moreover, this article also reviews the treatment of AHP-related comorbidities and complications, such as hyponatremia and posterior reversible encephalopathy syndrome. High glucose infusion is the main treatment method for AHP in China, and the improvement in diagnosis and increased attention to rare diseases in China has promoted the development of the diagnosis and treatment of AHP, and it is expected to explore more suitable treatment methods for AHP in the Chinese population in the future.
-
Key words:
- Porphyrias, Hepatic /
- Heme /
- Therapeutics
-
[1] STÖLZEL U, DOSS MO, SCHUPPAN D. Clinical guide and update on porphyrias[J]. Gastroenterology, 2019, 157( 2): 365- 381. DOI: 10.1053/j.gastro.2019.04.050. [2] Red Blood Cell Diseases(Anemia) Group of the Hematology Branch of the Chinese Medical Association. Expert consensus on the diagnosis and treatment of porphyrias in China(2020)[J]. Natl Med J China, 2020, 100( 14): 1051- 1056. DOI: 10.3760/cma.j.cn112137-20200219-00349.中华医学会血液学分会红细胞疾病(贫血)学组. 中国卟啉病诊治专家共识(2020年)[J]. 中华医学杂志, 2020, 100( 14): 1051- 1056. DOI: 10.3760/cma.j.cn112137-20200219-00349. [3] BUSTAD HJ, KALLIO JP, VORLAND M, et al. Acute intermittent Porphyria: An overview of therapy developments and future perspectives focusing on stabilisation of HMBS and proteostasis regulators[J]. Int J Mol Sci, 2021, 22( 2): 675. DOI: 10.3390/ijms22020675. [4] ANDERSON KE. Acute hepatic porphyrias: Current diagnosis& management[J]. Mol Genet Metab, 2019, 128( 3): 219- 227. DOI: 10.1016/j.ymgme.2019.07.002. [5] WANG B, BONKOVSKY HL, LIM JK, et al. AGA clinical practice update on diagnosis and management of acute hepatic porphyrias: Expert review[J]. Gastroenterology, 2023, 164( 3): 484- 491. DOI: 10.1053/j.gastro.2022.11.034. [6] WANG B, RUDNICK S, CENGIA B, et al. Acute hepatic porphyrias: Review and recent progress[J]. Hepatol Commun, 2018, 3( 2): 193- 206. DOI: 10.1002/hep4.1297. [7] YARRA P, FAUST D, BENNETT M, et al. Benefits of prophylactic heme therapy in severe acute intermittent porphyria[J]. Mol Genet Metab Rep, 2019, 19: 100450. DOI: 10.1016/j.ymgmr.2019.01.002. [8] ZÜBARIOĞLU T, KıYKıM E, AKTUĞLU-ZEYBEK Ç. An overview of acute hepatic porphyrias: Clinical implications, diagnostic approaches, and management strategies[J]. Turk Arch Pediatr, 2023, 58( 1): 3- 9. DOI: 10.5152/TurkArchPediatr.2022.22301. [9] PETRIDES PE. Therapy follows diagnosis: Old and new approaches for the treatment of acute porphyrias, what we know and what we should know[J]. Diagnostics, 2022, 12( 7): 1618. DOI: 10.3390/diagnostics12071618. [10] TRABER GM, YU AM. RNAi-based therapeutics and novel RNA bioengineering technologies[J]. J Pharmacol Exp Ther, 2023, 384( 1): 133- 154. DOI: 10.1124/jpet.122.001234. [11] YASUDA M, GAN L, CHEN B, et al. RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice[J]. Proc Natl Acad Sci U S A, 2014, 111( 21): 7777- 7782. DOI: 10.1073/pnas.1406228111. [12] SARDH E, HARPER P, BALWANI M, et al. Phase 1 trial of an RNA interference therapy for acute intermittent Porphyria[J]. N Engl J Med, 2019, 380( 6): 549- 558. DOI: 10.1056/NEJMoa1807838. [13] BALWANI M, SARDH E, VENTURA P, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent Porphyria[J]. N Engl J Med, 2020, 382( 24): 2289- 2301. DOI: 10.1056/NEJMoa1913147. [14] LEE J, MELCH M, ROBBIE GJ. Pharmacokinetic-pharmacodynamic model of urinary δ-aminolevulinic acid reduction after givosiran treatment in patients with acute hepatic porphyria[J]. CPT Pharmacometrics Syst Pharmacol, 2023, 12( 6): 842- 852. DOI: 10.1002/psp4.12957. [15] KUTER DJ, BONKOVSKY HL, MONROY S, et al. Efficacy and safety of givosiran for acute hepatic porphyria: Final results of the randomized phase III ENVISION trial[J]. J Hepatol, 2023, 79( 5): 1150- 1158. DOI: 10.1016/j.jhep.2023.06.013. [16] MA CD, FAUST D, BONKOVSKY HL. Idiosyncratic drug-induced liver injury caused by givosiran in a patient with acute intermittent porphyria[J]. Mol Genet Metab Rep, 2022, 34: 100946. DOI: 10.1016/j.ymgmr.2022.100946. [17] YASUDA M, KEEL S, BALWANI M. RNA interference therapy in acute hepatic porphyrias[J]. Blood, 2023, 142( 19): 1589- 1599. DOI: 10.1182/blood.2022018662. [18] Netherlands Alnylam. Givlaari: summary of product characteristics[EB/OL]. 2020. https://www.ema.europa.eu/en/documents/product-information/givlaari-epar-product-information_en.pdf. https://www.ema.europa.eu/en/documents/product-information/givlaari-epar-product-information_en.pdf [19] SARDH E, REJKJAER L, ANDERSSON DEH, et al. Safety, pharmacokinetics and pharmocodynamics of recombinant human porphobilinogen deaminase in healthy subjects and asymptomatic carriers of the acute intermittent porphyria gene who have increased porphyrin precursor excretion[J]. Clin Pharmacokinet, 2007, 46( 4): 335- 349. DOI: 10.2165/00003088-200746040-00006. [20] FONTANELLAS A, ÁVILA MA, BERRAONDO P. Emerging therapies for acute intermittent porphyria[J]. Expert Rev Mol Med, 2016, 18: e17. DOI: 10.1017/erm.2016.18. [21] CÓRDOBA KM, SERRANO-MENDIOROZ I, JERICÓ D, et al. Recombinant porphobilinogen deaminase targeted to the liver corrects enzymopenia in a mouse model of acute intermittent porphyria[J]. Sci Transl Med, 2022, 14( 627): eabc0700. DOI: 10.1126/scitranslmed.abc0700. [22] JERICÓ D, CÓRDOBA KM, SAMPEDRO A, et al. Recent insights into the pathogenesis of acute Porphyria attacks and increasing hepatic PBGD as an etiological treatment[J]. Life, 2022, 12( 11): 1858. DOI: 10.3390/life12111858. [23] LI R, REN Y, WANG JH, et al. Advances in the treatment of acute intermittent porphyria[J]. J Clin Hepatol, 2021, 37( 11): 2728- 2731. DOI: 10.3969/j.issn.1001-5256.2021.11.053.李茹, 任毅, 王建红, 等. 急性间歇性卟啉病的治疗及进展[J]. 临床肝胆病杂志, 2021, 37( 11): 2728- 2731. DOI: 10.3969/j.issn.1001-5256.2021.11.053. [24] PAÑEDA A, LOPEZ-FRANCO E, KAEPPEL C, et al. Safety and liver transduction efficacy of rAAV5-cohPBGD in nonhuman Primates: A potential therapy for acute intermittent porphyria[J]. Hum Gene Ther, 2013, 24( 12): 1007- 1017. DOI: 10.1089/hum.2013.166. [25] D’AVOLA D, LÓPEZ-FRANCO E, SANGRO B, et al. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria[J]. J Hepatol, 2016, 65( 4): 776- 783. DOI: 10.1016/j.jhep.2016.05.012. [26] SERRANO-MENDIOROZ I, SAMPEDRO A, SERNA N, et al. Bioengineered PBGD variant improves the therapeutic index of gene therapy vectors for acute intermittent porphyria[J]. Hum Mol Genet, 2018, 27( 21): 3688- 3696. DOI: 10.1093/hmg/ddy283. [27] SERRANO-MENDIOROZ I, SAMPEDRO A, ALEGRE M, et al. An inducible promoter responsive to different porphyrinogenic stimuli improves gene therapy vectors for acute intermittent Porphyria[J]. Hum Gene Ther, 2018, 29( 4): 480- 491. DOI: 10.1089/hum.2017.056. [28] CÓRDOBA KM, JERICÓ D, SAMPEDRO A, et al. Messenger RNA as a personalized therapy: The moment of truth for rare metabolic diseases[J]. Int Rev Cell Mol Biol, 2022, 372: 55- 96. DOI: 10.1016/bs.ircmb.2022.03.005. [29] JIANG L, BERRAONDO P, JERICÓ D, et al. Systemic messenger RNA as an etiological treatment for acute intermittent porphyria[J]. Nat Med, 2018, 24( 12): 1899- 1909. DOI: 10.1038/s41591-018-0199-z. [30] BUSTAD HJ, TOSKA K, SCHMITT C, et al. A pharmacological chaperone therapy for acute intermittent Porphyria[J]. Mol Ther, 2020, 28( 2): 677- 689. DOI: 10.1016/j.ymthe.2019.11.010. [31] HALLOY F, IYER PS, GHIDINI A, et al. Repurposing of glycine transport inhibitors for the treatment of erythropoietic protoporphyria[J]. Cell Chem Biol, 2021, 28( 8): 1221- 1234. DOI: 10.1016/j.chembiol.2021.02.021. [32] SOLARES I, TEJEDOR M, JERICÓ D, et al. Management of hyponatremia associated with acute porphyria-proposal for the use of tolvaptan[J]. Ann Transl Med, 2020, 8( 17): 1098. DOI: 10.21037/atm-20-1529. [33] MROZEK S, ROUSSET D, GEERAERTS T. Pharmacotherapy of sodium disorders in neurocritical care[J]. Curr Opin Crit Care, 2019, 25( 2): 132- 137. DOI: 10.1097/MCC.0000000000000589. [34] LI QY, REN Y, HOU JT, et al. Refractory hyponatremia caused by acute intermittent porphyria[J]. Chin J Endocrinol Metab, 2022, 38( 9): 815- 818. DOI: 10.3760/cma.j.cn311282-20211103-00699.李青阳, 任毅, 侯敬天, 等. 急性间歇性卟啉病所致顽固性低钠血症的临诊应对[J]. 中华内分泌代谢杂志, 2022, 38( 9): 815- 818. DOI: 10.3760/cma.j.cn311282-20211103-00699. [35] PERI A. Management of hyponatremia: Causes, clinical aspects, differential diagnosis and treatment[J]. Expert Rev Endocrinol Metab, 2019, 14( 1): 13- 21. DOI: 10.1080/17446651.2019.1556095. [36] SHANKAR J, BANFIELD J. Posterior reversible encephalopathy syndrome: A review[J]. Can Assoc Radiol J, 2017, 68( 2): 147- 153. DOI: 10.1016/j.carj.2016.08.005. [37] ZHENG XP, LIU XJ, WANG Y, et al. Acute intermittent porphyria presenting with seizures and posterior reversible encephalopathy syndrome: Two case reports and a literature review[J]. Medicine, 2018, 97( 36): e11665. DOI: 10.1097/MD.0000000000011665. [38] GOUYA L, VENTURA P, BALWANI M, et al. EXPLORE: A prospective, multinational, natural history study of patients with acute hepatic Porphyria with recurrent attacks[J]. Hepatology, 2020, 71( 5): 1546- 1558. DOI: 10.1002/hep.30936. [39] HAVERKAMP T, BRONISCH O, KNÖSEL T, et al. Heterogeneous molecular behavior in liver tumors(HCC and CCA) of two patients with acute intermittent porphyria[J]. J Cancer Res Clin Oncol, 2023, 149( 6): 2647- 2655. DOI: 10.1007/s00432-022-04384-5. [40] LISSING M, NOWAK G, ADAM R, et al. Liver transplantation for acute intermittent Porphyria[J]. Liver Transpl, 2021, 27( 4): 491- 501. DOI: 10.1002/lt.25959. [41] STORJORD E, AIRILA-MÅNSSON S, KARLSEN K, et al. Dental and periodontal health in acute intermittent Porphyria[J]. Life, 2022, 12( 8): 1270. DOI: 10.3390/life12081270. [42] MOORE AW 3rd, COKE JM. Acute porphyric disorders[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2000, 90( 3): 257- 262. DOI: 10.1067/moe.2000.107976. [43] GUO YY, LUO M. Treatment of acute intermittent porphyria related to menstrual cycle with GnRH-A[J]. J Reprod Med, 2023, 32( 3): 438- 442. DOI: 10.3969/j.issn.1004-3845.2023.03.022.郭瀛瀛, 罗敏. 与月经周期相关的急性间歇性卟啉病GnRH-a治疗探讨[J]. 生殖医学杂志, 2023, 32( 3): 438- 442. DOI: 10.3969/j.issn.1004-3845.2023.03.022. [44] XU J, YI CH, HE JP, et al. Long-term remission of acute intermittent Porphyria treated with gonadotropin-releasing hormone analogues and estrogen: A case report[J]. Clin Lab, 2022, 68( 9). DOI: 10.7754/Clin.Lab.2022.211218. [45] TSUDA M, OGAWA K, ENDOU T, et al. Absorption, metabolism, and excretion of[14C]dersimelagon, an investigational oral selective melanocortin 1 receptor agonist, in preclinical species and healthy volunteers[J]. Pharmacol Res Perspect, 2023, 11( 3): e01084. DOI: 10.1002/prp2.1084. -



 PDF下载 ( 976 KB)
PDF下载 ( 976 KB)

 下载:
下载:


