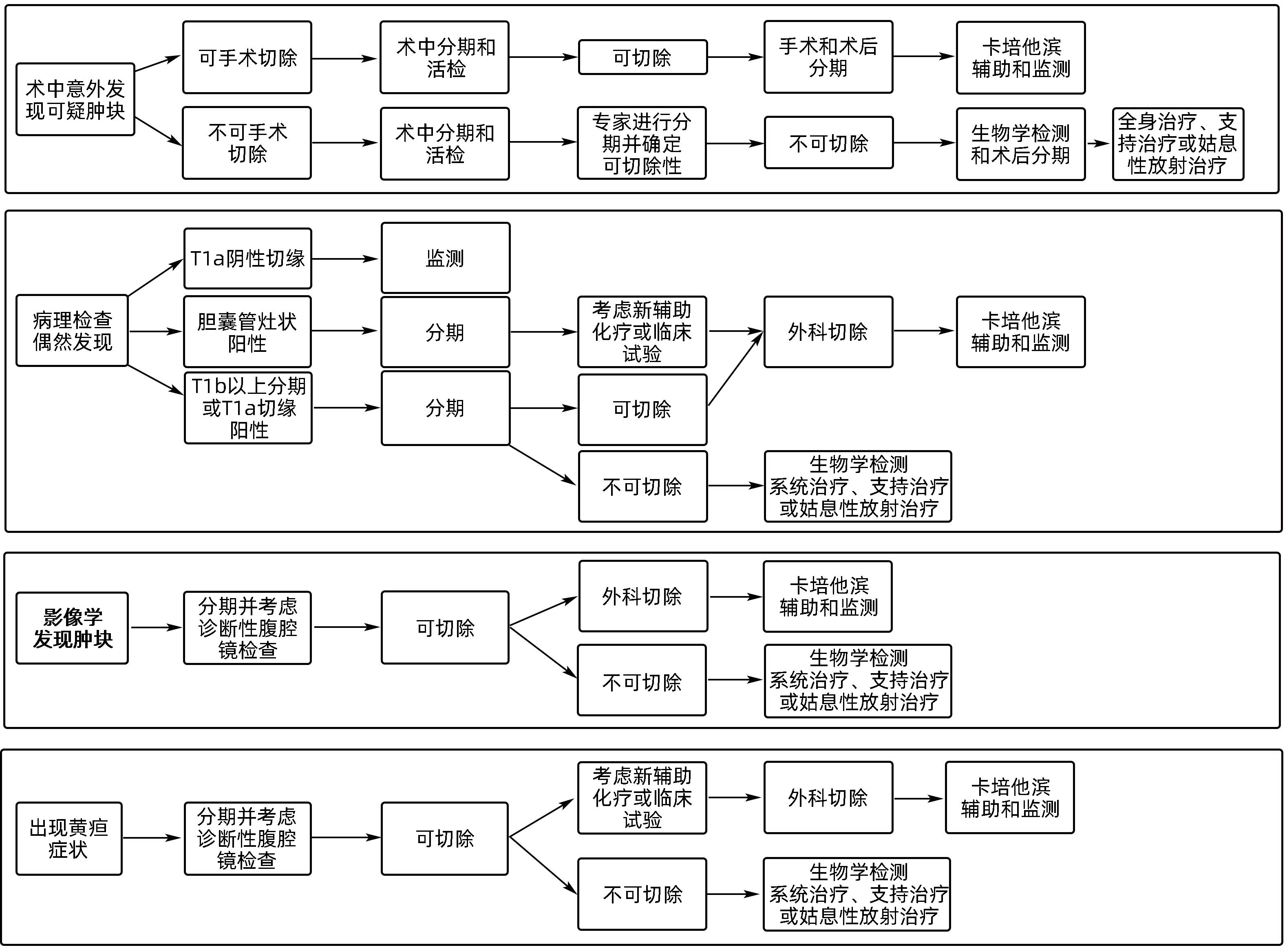
《肿瘤外科学年鉴: 肝外胆管癌和胆囊癌临床诊疗指南》推荐意见
DOI: 10.12449/JCH240407
Recommendations from Annals of Surgical Oncology: Clinical guidelines for extrahepatic cholangiocarcinoma and gallbladder carcinoma
-
摘要: 胆道恶性肿瘤是一类发病率低但侵袭性强的消化道肿瘤,主要包括肝内胆管癌、肝外胆管癌和胆囊癌,常常伴随着局部进展或远处转移等特征。对于局部可切除的患者而言,手术往往是首选的治疗方法。然而,即便患者接受根治性手术其术后复发风险依然很高。因此,对于胆道恶性肿瘤患者而言,通常需要采取多种治疗模式,包括手术切除、全身治疗(如靶向治疗、化学治疗、免疫治疗)以及/或局部治疗的综合方案。随着胆道恶性肿瘤领域的逐渐发展,对于外科肿瘤学家而言,了解并掌握最新的外科诊疗策略以及最佳患者的选择和管理体系至关重要。鉴于治疗的复杂性和诊疗技术不断发展的特点,美国肿瘤外科学权威期刊《肿瘤外科学年鉴》于近期发表了关于肝胆肿瘤的实践诊疗指南,主要包括肝细胞癌、肝内胆管癌、肝外胆管癌和胆囊癌,旨在为肝胆肿瘤患者的临床管理和决策制定提供更多基于循证医学的证据。限于篇幅和不同侧重点,本文着重介绍该指南中关于肝外胆管癌和胆囊癌的评估要点和临床治疗的相关建议,以供临床参考。Abstract: Biliary tract carcinoma (BTC) is a type of gastrointestinal tumor with a low incidence rate and a strong invasive ability, mainly including intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (ECC), and gallbladder carcinoma (GC), often accompanied by local progression or distant metastasis. Surgery is often the preferred treatment method for patients with local resectable tumor; however, there is still a high risk of recurrence after radical surgery. Therefore, multiple treatment modalities are often required for BTC patients, including surgical resection, systemic treatment (such as targeted therapy, chemotherapy, and immunotherapy), and/or a combination of local treatment methods. With the development of the field of BTC, it is critical for surgical oncologists to understand and master the latest surgical strategies and the best patient selection and management systems. In view of the complexity of treatment and the continuous development of diagnosis and treatment techniques, Annals of Surgical Oncology, an authoritative American journal of cancer surgery, recently published the practical diagnosis and treatment guidelines for hepatobiliary tumors, including hepatocellular carcinoma (HCC), ICC, ECC, and GC, aiming to provide more evidence-based evidence for the clinical management and decision-making of patients with hepatobiliary tumors. Due to the limitations of length and different emphases, this article mainly introduces the recommendations for the evaluation points and clinical treatment of ECC and GC in the guidelines, so as to provide a reference for clinical practice.
-
表 1 ECC和GC的手术治疗和围手术期管理注意事项的对比
Table 1. Comparison of surgical and perioperative management considerations for ECC and GC
注意事项 ECC GC 手术禁忌证 绝对:肝门外淋巴结转移;FLR不足(如果需要切除肝脏);晚期肝硬化;肝外转移 相对:出现黄疸,预后差 绝对:肝门外淋巴结转移;肝外疾病 手术计划 肿瘤的解剖学评估; 肝容量指标(如适合手术); 术前胆道引流; 肿瘤的解剖学评估 腹腔镜检查 选择(联合肝脏超声) 选择(联合肝脏/胆道超声) 术式选择 肝门部肿瘤:视解剖情况而定; 胆管切除+/-肝切除; 远端肿瘤:胆管切除术或Whipple手术 T1b及以上和淋巴结阳性者需要行胆囊切除术和肝4b/5段切除; 意外GC,术后病理分期在T1b以上者需要在初次术后4~8周再次行肝4b/5段切除 淋巴结清扫 清扫(6枚淋巴结) 清扫(6枚淋巴结) 边缘距离 没有一致意见,建议R0边缘; 肝门部胆管癌切除应包括尾状叶以提高阴性切缘率; 标本近端和远端应做冷冻病理切片 没有一致意见,建议R0切缘; 胆囊管切缘需做冷冻病理切片检查 术前新辅助化疗 手术可切除者目前没有建议 手术可切除者目前没有建议; 对于T2或T3分期肿瘤的新辅助治疗方案目前正在试验中 术后辅助化疗 卡培他滨 卡培他滨 注:FLR,残余肝脏体积。 -
[1] ONG AML. Outrunning burnout in a GI fellowship program during the COVID-19 pandemic[J]. Dig Dis Sci, 2020, 65( 8): 2161- 2163. DOI: 10.1007/s10620-020-06401-4. [2] KUDO M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma[J]. Hepatobiliary Surg Nutr, 2022, 11( 4): 592- 596. DOI: 10.21037/hbsn-22-143. [3] LEE AJ, CHUN YS. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates[J]. Chin Clin Oncol, 2018, 7( 5): 52. DOI: 10.21037/cco.2018.07.03. [4] CARDINALE V, SEMERARO R, TORRICE A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors[J]. World J Gastrointest Oncol, 2010, 2( 11): 407- 416. DOI: 10.4251/wjgo.v2.i11.407. [5] WRONKA KM, GRĄT M, STYPUŁKOWSKI J, et al. Relevance of preoperative hyperbilirubinemia in patients undergoing hepatobiliary resection for hilar cholangiocarcinoma[J]. J Clin Med, 2019, 8( 4): 458. DOI: 10.3390/jcm8040458. [6] VOGEL A, BRIDGEWATER J, EDELINE J, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2023, 34( 2): 127- 140. DOI: 10.1016/j.annonc.2022.10.506. [7] KELLEY RK, UENO M, YOO C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer(KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2023, 401( 10391): 1853- 1865. DOI: 10.1016/S0140-6736(23)00727-4. [8] DUMONCEAU JM, TRINGALI A, PAPANIKOLAOU IS, et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy(ESGE) Clinical Guideline-Updated October 2017[J]. Endoscopy, 2018, 50( 9): 910- 930. DOI: 10.1055/a-0659-9864. [9] MANSOUR JC, ALOIA TA, CRANE CH, et al. Hilar cholangiocarcinoma: Expert consensus statement[J]. HPB, 2015, 17( 8): 691- 699. DOI: 10.1111/hpb.12450. [10] UEMURA K, MURAKAMI Y, SATOI S, et al. Impact of preoperative biliary drainage on long-term survival in resected pancreatic ductal adenocarcinoma: A multicenter observational study[J]. Ann Surg Oncol, 2015, 22( Suppl 3): S1238- S1246. DOI: 10.1245/s10434-015-4618-9. [11] STROM TJ, KLAPMAN JB, SPRINGETT GM, et al. Comparative long-term outcomes of upfront resected pancreatic cancer after preoperative biliary drainage[J]. Surg Endosc, 2015, 29( 11): 3273- 3281. DOI: 10.1007/s00464-015-4075-3. [12] MIURA F, SANO K, WADA K, et al. Prognostic impact of type of preoperative biliary drainage in patients with distal cholangiocarcinoma[J]. Am J Surg, 2017, 214( 2): 256- 261. DOI: 10.1016/j.amjsurg.2017.01.010. [13] TIEU AH, KUMBHARI V, JAKHETE N, et al. Diagnostic and therapeutic utility of SpyGlass(®) peroral cholangioscopy in intraductal biliary disease: Single-center, retrospective, cohort study[J]. Dig Endosc, 2015, 27( 4): 479- 485. DOI: 10.1111/den.12405. [14] PEREIRA P, VILAS-BOAS F, PEIXOTO A, et al. How SpyGlass™ may impact endoscopic retrograde cholangiopancreatography practice and patient management[J]. GE Port J Gastroenterol, 2018, 25( 3): 132- 137. DOI: 10.1159/000481859. [15] GÓMEZ-ESPAÑA MA, MONTES AF, GARCIA-CARBONERO R, et al. SEOM clinical guidelines for pancreatic and biliary tract cancer(2020)[J]. Clin Transl Oncol, 2021, 23( 5): 988- 1000. DOI: 10.1007/s12094-021-02573-1. [16] GOERE D, WAGHOLIKAR GD, PESSAUX P, et al. Utility of staging laparoscopy in subsets of biliary cancers: Laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma[J]. Surg Endosc, 2006, 20( 5): 721- 725. DOI: 10.1007/s00464-005-0583-x. [17] PRIMROSE JN, FOX RP, PALMER DH, et al. Capecitabine compared with observation in resected biliary tract cancer(BILCAP): A randomised, controlled, multicentre, phase 3 study[J]. Lancet Oncol, 2019, 20( 5): 663- 673. DOI: 10.1016/S1470-2045(18)30915-X. [18] BANALES JM, MARIN JJG, LAMARCA A, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17( 9): 557- 588. DOI: 10.1038/s41575-020-0310-z. [19] SOARES KC, KAMEL I, COSGROVE DP, et al. Hilar cholangiocarcinoma: Diagnosis, treatment options, and management[J]. Hepatobiliary Surg Nutr, 2014, 3( 1): 18- 34. DOI: 10.3978/j.issn.2304-3881.2014.02.05. [20] BHUTIANI N, SCOGGINS CR, MCMASTERS KM, et al. The impact of caudate lobe resection on margin status and outcomes in patients with hilar cholangiocarcinoma: A multi-institutional analysis from the US Extrahepatic Biliary Malignancy Consortium[J]. Surgery, 2018, 163( 4): 726- 731. DOI: 10.1016/j.surg.2017.10.028. [21] WAHAB MA, SULTAN AM, SALAH T, et al. Caudate lobe resection with major hepatectomy for central cholangiocarcinoma: Is it of value?[J]. Hepato-gastroenterology, 2012, 59( 114): 321- 324. DOI: 10.5754/hge11999. [22] WEBER SM, RIBERO D, O’REILLY EM, et al. Intrahepatic cholangiocarcinoma: Expert consensus statement[J]. HPB, 2015, 17( 8): 669- 680. DOI: 10.1111/hpb.12441. [23] HEIMBACH JK, GORES GJ, HADDOCK MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma[J]. Semin Liver Dis, 2004, 24( 2): 201- 207. DOI: 10.1055/s-2004-828896. [24] BREUER E, MUELLER M, DOYLE MB, et al. Liver transplantation as a new standard of care in patients with perihilar cholangiocarcinoma? Results from an international benchmark study[J]. Ann Surg, 2022, 276( 5): 846- 853. DOI: 10.1097/SLA.0000000000005641. [25] ETHUN CG, LOPEZ-AGUIAR AG, ANDERSON DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma: An argument for shifting treatment paradigms for resectable disease[J]. Ann Surg, 2018, 267( 5): 797- 805. DOI: 10.1097/SLA.0000000000002574. [26] PARK JH, CHOI EK, AHN SD, et al. Postoperative chemoradiotherapy for extrahepatic bile duct cancer[J]. Int J Radiat Oncol Biol Phys, 2011, 79( 3): 696- 704. DOI: 10.1016/j.ijrobp.2009.12.031. [27] BORGHERO Y, CRANE CH, SZKLARUK J, et al. Extrahepatic bile duct adenocarcinoma: Patients at high-risk for local recurrence treated with surgery and adjuvant chemoradiation have an equivalent overall survival to patients with standard-risk treated with surgery alone[J]. Ann Surg Oncol, 2008, 15( 11): 3147- 3156. DOI: 10.1245/s10434-008-9998-7. [28] KIM TH, HAN SS, PARK SJ, et al. Role of adjuvant chemoradiotherapy for resected extrahepatic biliary tract cancer[J]. Int J Radiat Oncol Biol Phys, 2011, 81( 5): e853- e859. DOI: 10.1016/j.ijrobp.2010.12.019. [29] LIM KH, OH DY, CHIE EK, et al. Adjuvant concurrent chemoradiation therapy(CCRT) alone versus CCRT followed by adjuvant chemotherapy: Which is better in patients with radically resected extrahepatic biliary tract cancer: A non-randomized, single center study[J]. BMC Cancer, 2009, 9: 345. DOI: 10.1186/1471-2407-9-345. [30] NELSON JW, GHAFOORI AP, WILLETT CG, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma[J]. Int J Radiat Oncol Biol Phys, 2009, 73( 1): 148- 153. DOI: 10.1016/j.ijrobp.2008.07.008. [31] HUGHES MA, FRASSICA DA, YEO CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct[J]. Int J Radiat Oncol Biol Phys, 2007, 68( 1): 178- 182. DOI: 10.1016/j.ijrobp.2006.11.048. [32] BEN-JOSEF E, GUTHRIE KA, EL-KHOUEIRY AB, et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma[J]. J Clin Oncol, 2015, 33( 24): 2617- 2622. DOI: 10.1200/JCO.2014.60.2219. [33] HAWKINS WG, DEMATTEO RP, JARNAGIN WR, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer[J]. Ann Surg Oncol, 2004, 11( 3): 310- 315. DOI: 10.1245/aso.2004.03.011. [34] DASARI BVM, IONESCU MI, PAWLIK TM, et al. Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: A systematic review and meta-analysis[J]. J Surg Oncol, 2018, 118( 3): 477- 485. DOI: 10.1002/jso.25186. [35] NISHIO H, EBATA T, YOKOYAMA Y, et al. Gallbladder cancer involving the extrahepatic bile duct is worthy of resection[J]. Ann Surg, 2011, 253( 5): 953- 960. DOI: 10.1097/SLA.0b013e318216f5f3. [36] REGIMBEAU JM, FUKS D, BACHELLIER P, et al. Prognostic value of jaundice in patients with gallbladder cancer by the AFC-GBC-2009 study group[J]. Eur J Surg Oncol, 2011, 37( 6): 505- 512. DOI: 10.1016/j.ejso.2011.03.135. [37] ALOIA TA, JÁRUFE N, JAVLE M, et al. Gallbladder cancer: Expert consensus statement[J]. HPB, 2015, 17( 8): 681- 690. DOI: 10.1111/hpb.12444. [38] FUKS D, REGIMBEAU JM, LE TREUT YP, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group[J]. World J Surg, 2011, 35( 8): 1887- 1897. DOI: 10.1007/s00268-011-1134-3. [39] LEE SE, JANG JY, LIM CS, et al. Systematic review on the surgical treatment for T1 gallbladder cancer[J]. World J Gastroenterol, 2011, 17( 2): 174- 180. DOI: 10.3748/wjg.v17.i2.174. [40] QADAN M, KINGHAM TP. Technical aspects of gallbladder cancer surgery[J]. Surg Clin North Am, 2016, 96( 2): 229- 245. DOI: 10.1016/j.suc.2015.12.007. [41] RUFF SM, CLOYD JM, PAWLIK TM. Annals of surgical oncology practice guidelines series: Management of primary liver and biliary tract cancers[J]. Ann Surg Oncol, 2023, 30( 13): 7935- 7949. DOI: 10.1245/s10434-023-14255-z. -



 PDF下载 ( 997 KB)
PDF下载 ( 997 KB)

 下载:
下载: