外泌体在肝内胆管癌中的作用
DOI: 10.12449/JCH240130
-
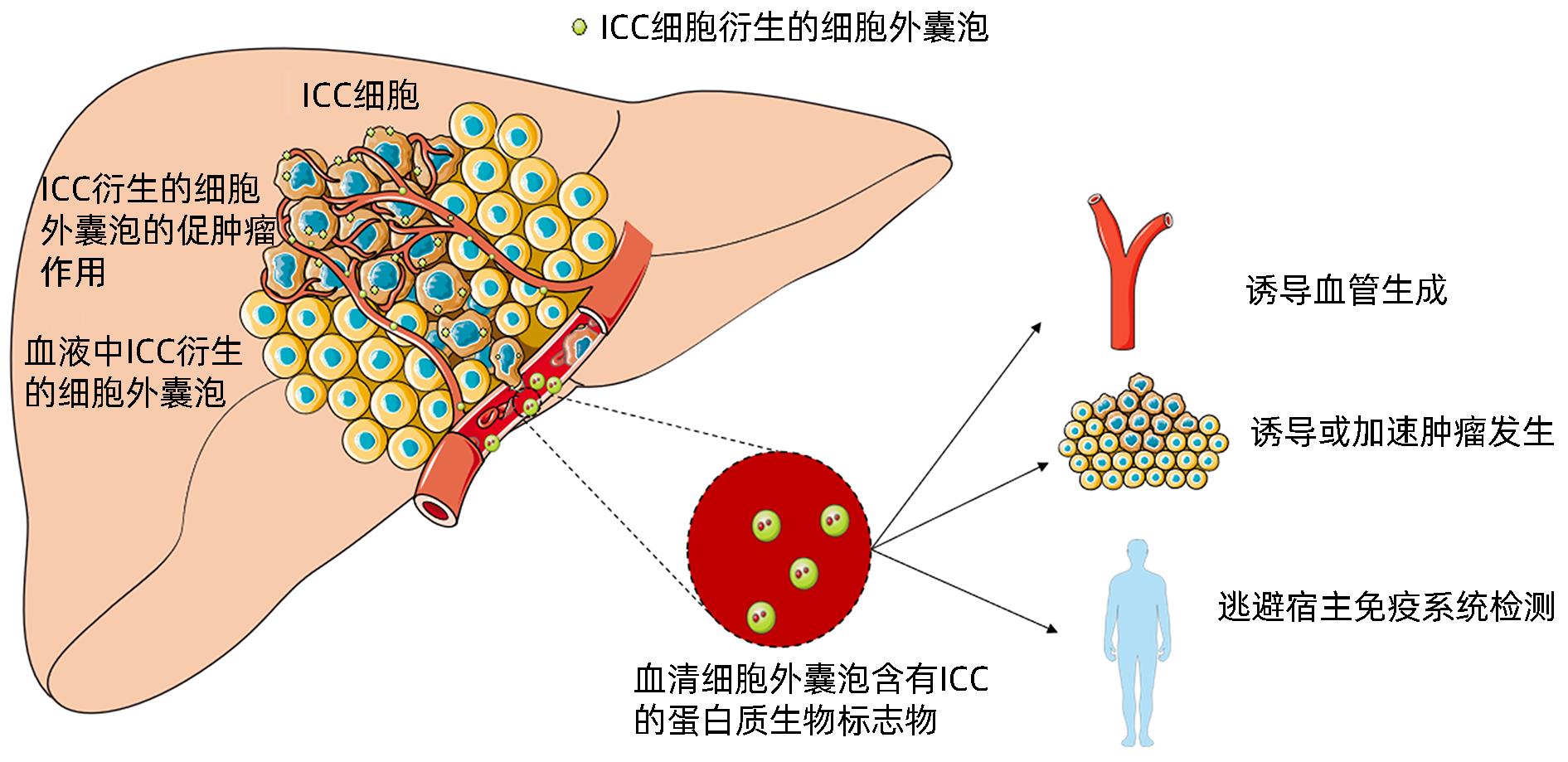
摘要: 肝内胆管癌(ICC)是一种特殊类型的肝癌,其早期临床症状不典型,大多数患者初诊时已处于中晚期。由于缺乏有效的分子标志物和治疗手段,ICC患者5年生存率极低。外泌体是一种细胞分泌的囊泡,包含蛋白质、RNA、脂质等,是细胞间通讯的重要载体。近期研究显示外泌体在ICC发生发展过程中扮演重要角色,本文就外泌体在ICC中的诊断、治疗作用及其机制进行综述,并展望外泌体的治疗前景与潜在的临床应用。Abstract: Intrahepatic cholangiocarcinoma (ICC) is a special type of liver cancer with atypical clinical symptoms in the early stage, and most patients are already in the advanced stage at the time of initial diagnosis. Due to a lack of effective molecular markers and treatment options, ICC patients tend to have an extremely low five-year survival rate. Exosomes are vesicles secreted by cells that contain proteins, RNA, and lipids, and they are important carriers of intercellular communication. Recent studies have shown that exosomes play a crucial role in the development and progression of ICC, and this article reviews the role and mechanism of exosomes in the diagnosis and treatment of ICC and looks into the future treatment prospect and potential clinical application of exosomes.
-
Key words:
- Cholangiocarcinoma /
- Exosomes /
- Diagnosis /
- Therapeutics
-
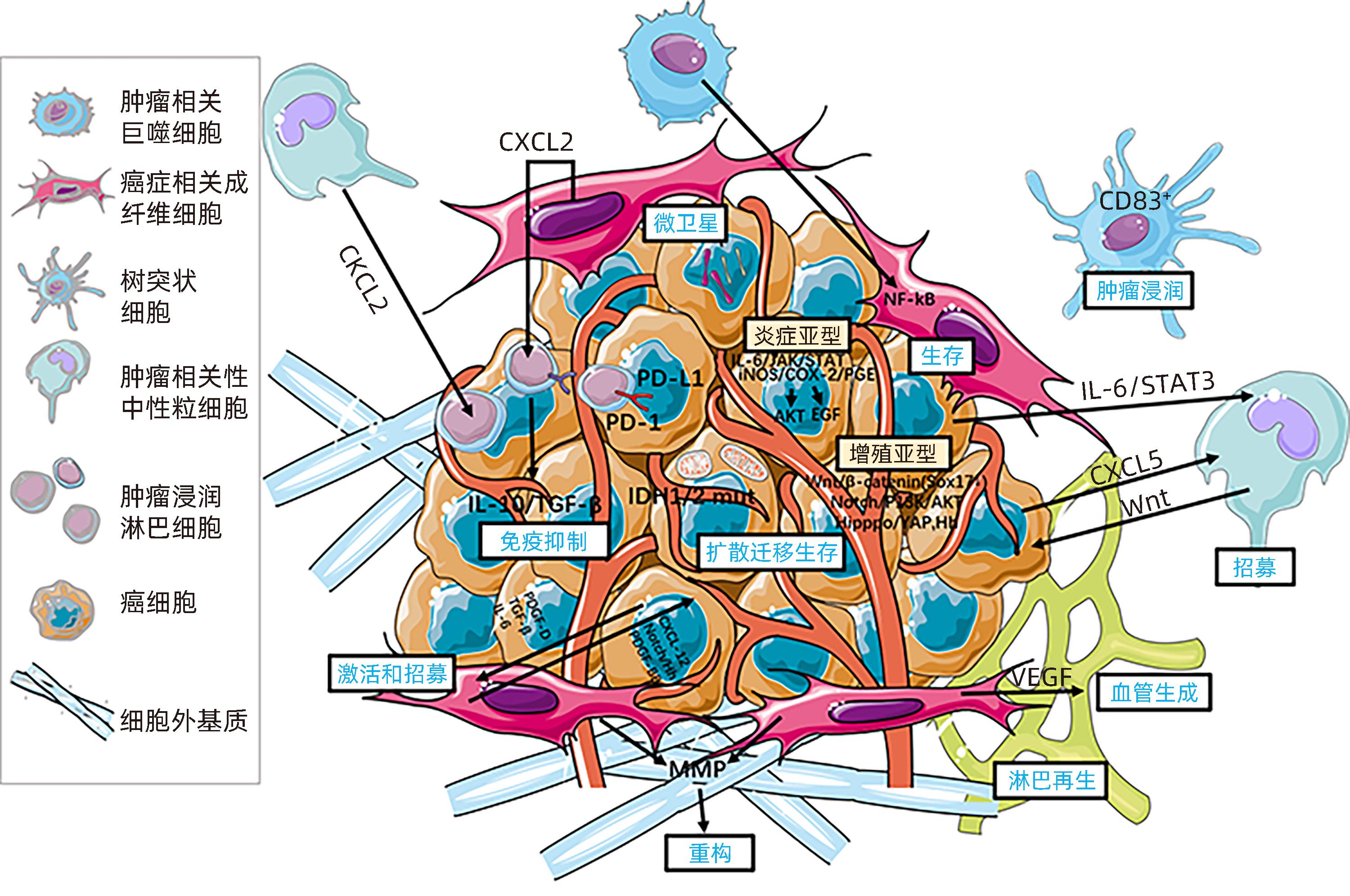
注: EVCOX,环加氧酶;CTLA-4,细胞毒性T淋巴细胞抗原4;CXCL,C-X-C基序趋化因子配体;EGF,表皮生长因子;ERK,细胞外信号调节激酶;Hh,刺猬通路;IDH,异柠檬酸脱氢酶;iNOS,诱导氮氧化物合酶;MMP,基质金属蛋白酶;NF-κB,核因子κB;PDGF,血小板衍生生长因子;PD-1,程序性死亡蛋白1;PD-L1,程序性死亡配体1;PGE,前列腺素E;PI3K,磷脂酰肌醇3-激酶;STAT3,信号传导和转录因子3;TAM,肿瘤相关巨噬细胞;TAN,肿瘤相关性中性粒细胞。
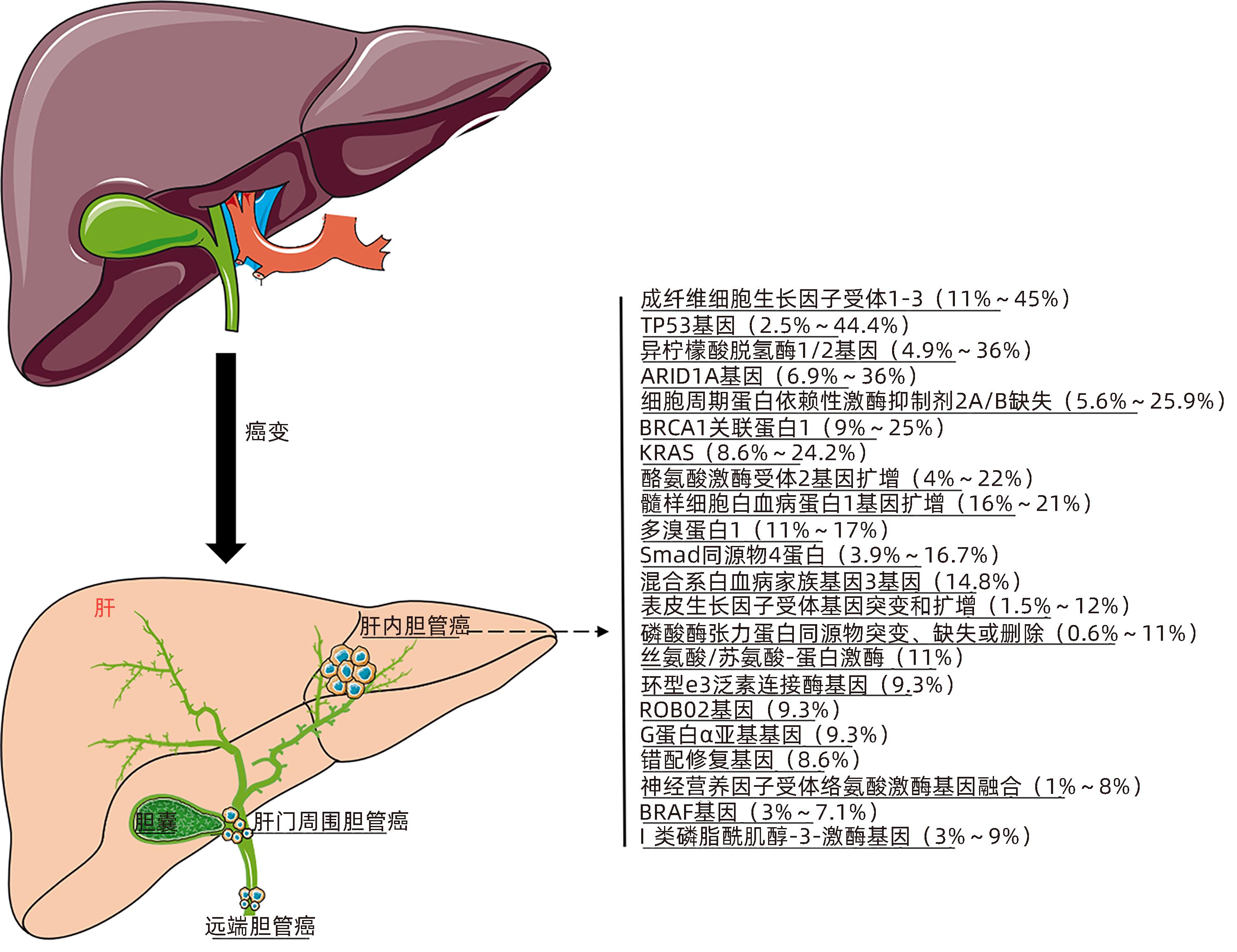
图 2 ICC的分子发病机制
Figure 2. Molecular pathogenesis of cholangiocarcinoma
表 1 外泌体与其他传统细胞外囊泡的区别
Table 1. Differences between exosomes and other traditional extracellular vesicles
特征 直径(nm) 密度(g/mL) 形状 组成 标志物 细胞内起源 外泌体 40~100 1.13~1.19 杯状 胆固醇,鞘磷脂,神经酰胺,脂筏,暴露PPS CD63,CD9,Alix, TSG101 内部隔室 (核内体) 微泡 100~1 000 不规则形状 暴露PPS 整合素,选择素 和CD40配体 质膜 核外颗粒体 50~200 双圆形 胆固醇,二酰基甘油,PPS CR1和蛋白水解酶 质膜 膜颗粒 50~80 1.04~1.07 圆形 CD133 质膜 外泌体样颗粒 20~50 1.1 不规则形状 无脂筏 TNFR I 凋亡小泡 50~500 1.16~1.28 异形 组蛋白 -
[1] YANG KG, WANG WW, WANG Y, et al. Proteomic analysis of serum and serum exosomes, and their application in intrahepatic cholangiocarcinoma[J]. Chin J Chromatogr, 2021, 39( 11): 1191- 1202. DOI: 10.3724/SP.J.1123.2021.04009.杨凯歌, 王薇薇, 王彦, 等. 血清和血清外泌体的蛋白质组分析及其在肝内胆管癌中的应用[J]. 色谱, 2021, 39( 11): 1191- 1202. DOI: 10.3724/SP.J.1123.2021.04009. [2] CAO LP, HONG JW, WU J. Potential of extracellular vesicles and exosomes as diagnostic markers for cholangiocarcinoma[J]. Hepatobiliary Surg Nutr, 2022, 11( 3): 436- 438. DOI: 10.21037/hbsn-2022-02. [3] ARAI Y, TOTOKI Y, HOSODA F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma[J]. Hepatology, 2014, 59( 4): 1427- 1434. DOI: 10.1002/hep.26890. [4] SIA D, HOSHIDA Y, VILLANUEVA A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes[J]. Gastroenterology, 2013, 144( 4): 829- 840. DOI: 10.1053/j.gastro.2013.01.001. [5] ANDERSEN JB, SPEE B, BLECHACZ BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors[J]. Gastroenterology, 2012, 142( 4): 1021- 1031.e15. DOI: 10.1053/j.gastro.2011.12.005. [6] NEPAL C, O’ROURKE CJ, OLIVEIRA DVNP, et al. Genomic perturbations reveal distinct regulatory networks in intrahepatic cholangiocarcinoma[J]. Hepatology, 2018, 68( 3): 949- 963. DOI: 10.1002/hep.29764. [7] LIN JZ, SHI JP, GUO HL, et al. Alterations in DNA damage repair genes in primary liver cancer[J]. Clin Cancer Res, 2019, 25( 15): 4701- 4711. DOI: 10.1158/1078-0432.CCR-19-0127. [8] MAYNARD H, STADLER ZK, BERGER MF, et al. Germline alterations in patients with biliary tract cancers: A spectrum of significant and previously underappreciated findings[J]. Cancer, 2020, 126( 9): 1995- 2002. DOI: 10.1002/cncr.32740. [9] JUSAKUL A, CUTCUTACHE I, YONG CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma[J]. Cancer Discov, 2017, 7( 10): 1116- 1135. DOI: 10.1158/2159-8290.CD-17-0368. [10] CHAN-ON W, NAIRISMÄGI ML, ONG CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers[J]. Nat Genet, 2013, 45( 12): 1474- 1478. DOI: 10.1038/ng.2806. [11] ONG CK, SUBIMERB C, PAIROJKUL C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma[J]. Nat Genet, 2012, 44( 6): 690- 693. DOI: 10.1038/ng.2273. [12] RODRIGUES PM, OLAIZOLA P, PAIVA NA, et al. Pathogenesis of cholangiocarcinoma[J]. Annu Rev Pathol, 2021, 16: 433- 463. DOI: 10.1146/annurev-pathol-030220-020455. [13] XING C, LI H, LI RJ, et al. The roles of exosomal immune checkpoint proteins in tumors[J]. Mil Med Res, 2021, 8( 1): 56. DOI: 10.1186/s40779-021-00350-3. [14] HELWA I, CAI JW, DREWRY MD, et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents[J]. PLoS One, 2017, 12( 1): e0170628. DOI: 10.1371/journal.pone.0170628. [15] LOBB RJ, BECKER M, WEN SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma[J]. J Extracell Vesicles, 2015, 4: 27031. DOI: 10.3402/jev.v4.27031. [16] HMMIER A, O’BRIEN ME, LYNCH V, et al. Proteomic analysis of bronchoalveolar lavage fluid(BALF) from lung cancer patients using label-free mass spectrometry[J]. BBA Clin, 2017, 7: 97- 104. DOI: 10.1016/j.bbacli.2017.03.001. [17] YÁÑEZ-MÓ M, SILJANDER PRM, ANDREU Z, et al. Biological properties of extracellular vesicles and their physiological functions[J]. J Extracell Vesicles, 2015, 4: 27066. DOI: 10.3402/jev.v4.27066. [18] GUO J, ZHONG XX, TAN QL, et al. miR-301a-3p induced by endoplasmic reticulum stress mediates the occurrence and transmission of trastuzumab resistance in HER2-positive gastric cancer[J]. Cell Death Dis, 2021, 12( 7): 696. DOI: 10.1038/s41419-021-03991-3. [19] ZHOU DJ, GE W, CAO DD. Advances in the study of exosomes in the development and diagnosis of hepatocellular liver cancer[J]. Chin Med Herald, 2023, 20( 10): 42- 44, 54. DOI: 10.20047/j.issn1673-7210.2023.10.08.周丁杰, 戈伟, 曹德东. 外泌体在肝细胞肝癌发展和诊断中的研究进展[J]. 中国医药导报, 2023, 20( 10): 42- 44, 54. DOI: 10.20047/j.issn1673-7210.2023.10.08. [20] WEERAPHAN C, PHONGDARA A, CHAIYAWAT P, et al. Phosphoproteome profiling of isogenic cancer cell-derived exosome reveals HSP90 as a potential marker for human cholangiocarcinoma[J]. Proteomics, 2019, 19( 12): e1800159. DOI: 10.1002/pmic.201800159. [21] IKEDA C, HAGA H, MAKINO N, et al. Utility of Claudin-3 in extracellular vesicles from human bile as biomarkers of cholangiocarcinoma[J]. Sci Rep, 2021, 11( 1): 1195. DOI: 10.1038/s41598-021-81023-y. [22] ARBELAIZ A, AZKARGORTA M, KRAWCZYK M, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma[J]. Hepatology, 2017, 66( 4): 1125- 1143. DOI: 10.1002/hep.29291. [23] PAN Y, SHAO SJ, SUN H, et al. Bile-derived exosome noncoding RNAs as potential diagnostic and prognostic biomarkers for cholangiocarcinoma[J]. Front Oncol, 2022, 12: 985089. DOI: 10.3389/fonc.2022.985089. [24] HAN JY, AHN KS, KIM YH, et al. Circulating microRNAs as biomarkers in bile-derived exosomes of cholangiocarcinoma[J]. Ann Surg Treat Res, 2021, 101( 3): 140- 150. DOI: 10.4174/astr.2021.101.3.140. [25] GAO LL, YANG XP, ZHANG H, et al. Inhibition of miR-10a-5p suppresses cholangiocarcinoma cell growth through downregulation of Akt pathway[J]. Onco Targets Ther, 2018, 11: 6981- 6994. DOI: 10.2147/OTT.S182225. [26] LI O, JIANG B, YI WM, et al. LncRNA NEAT1 promotes cell proliferation, migration, and invasion via the miR-186-5p/PTP4A1 axis in cholangiocarcinoma[J]. Kaohsiung J Med Sci, 2021, 37( 5): 379- 391. DOI: 10.1002/kjm2.12354. [27] SUN ZP, TAN ZG, PENG C, et al. LncRNA SNHG3 facilitates the malignant phenotype of cholangiocarcinoma cells via the miR-3173-5p/ERG axis[J]. J Gastrointest Surg, 2022, 26( 4): 802- 812. DOI: 10.1007/s11605-021-05160-5. [28] CHEN Q, WANG HB, LI Z, et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription[J]. J Hepatol, 2022, 76( 1): 135- 147. DOI: 10.1016/j.jhep.2021.08.027. [29] CHEN HW, CHENGALVALA V, HU HX, et al. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis[J]. Acta Pharm Sin B, 2021, 11( 8): 2136- 2149. DOI: 10.1016/j.apsb.2021.04.012. [30] LIU JY, REN LW, LI S, et al. The biology, function, and applications of exosomes in cancer[J]. Acta Pharm Sin B, 2021, 11( 9): 2783- 2797. DOI: 10.1016/j.apsb.2021.01.001. [31] LI L, ZHAO J, ZHANG QB, et al. Cancer cell-derived exosomes promote HCC tumorigenesis through hedgehog pathway[J]. Front Oncol, 2021, 11: 756205. DOI: 10.3389/fonc.2021.756205. [32] WANG YZ, YI J, CHEN XG, et al. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-β and IL-10[J]. Oncol Lett, 2016, 11( 2): 1527- 1530. DOI: 10.3892/ol.2015.4044. [33] LI XX, WANG SH, ZHU RJ, et al. Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFκB-TLR signaling pathway[J]. J Hematol Oncol, 2016, 9: 42. DOI: 10.1186/s13045-016-0269-y. [34] LIN LY, DU LM, CAO K, et al. Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities[J]. Oncogene, 2016, 35( 46): 6038- 6042. DOI: 10.1038/onc.2016.131. [35] BECKER A, THAKUR BK, WEISS JM, et al. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis[J]. Cancer Cell, 2016, 30( 6): 836- 848. DOI: 10.1016/j.ccell.2016.10.009. [36] HE LQ, ZHU W, CHEN Q, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis[J]. Theranostics, 2019, 9( 26): 8206- 8220. DOI: 10.7150/thno.37455. [37] TANG MKS, YUE PYK, IP PP, et al. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface[J]. Nat Commun, 2018, 9( 1): 2270. DOI: 10.1038/s41467-018-04695-7. [38] PARK JE, DUTTA B, TSE SW, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift[J]. Oncogene, 2019, 38( 26): 5158- 5173. DOI: 10.1038/s41388-019-0782-x. [39] ZHANG PF, GAO C, HUANG XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma[J]. Mol Cancer, 2020, 19( 1): 110. DOI: 10.1186/s12943-020-01222-5. [40] XIE FT, XU MX, LU J, et al. The role of exosomal PD-L1 in tumor progression and immunotherapy[J]. Mol Cancer, 2019, 18( 1): 146. DOI: 10.1186/s12943-019-1074-3. [41] YIN Z, YU M, MA TT, et al. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: A key role of exosomal PD-L1[J]. J Immunother Cancer, 2021, 9( 1): e001698. DOI: 10.1136/jitc-2020-001698. [42] MOON B, CHANG S. Exosome as a delivery vehicle for cancer therapy[J]. Cells, 2022, 11( 3): 316. DOI: 10.3390/cells11030316. [43] WALKER S, BUSATTO S, PHAM A, et al. Extracellular vesicle-based drug delivery systems for cancer treatment[J]. Theranostics, 2019, 9( 26): 8001- 8017. DOI: 10.7150/thno.37097. [44] LI L, PIONTEK K, ISHIDA M, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model[J]. Hepatology, 2017, 65( 2): 501- 514. DOI: 10.1002/hep.28735. [45] SHA M, JEONG S, QIU BJ, et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma[J]. Cancer Med, 2018, 7( 9): 4665- 4677. DOI: 10.1002/cam4.1704. [46] SIRICA AE, CAMPBELL DJ, DUMUR CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma[J]. Curr Opin Gastroenterol, 2011, 27( 3): 276- 284. DOI: 10.1097/MOG.0b013e32834405c3. [47] FABRIS L, SATO K, ALPINI G, et al. The tumor microenvironment in cholangiocarcinoma progression[J]. Hepatology, 2021, 73( Suppl 1): 75- 85. DOI: 10.1002/hep.31410. [48] YANG RJ, WANG D, HAN S, et al. Erratum: miR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAF: Erratum[J]. Int J Biol Sci, 2022, 18( 11): 4466- 4467. DOI: 10.7150/ijbs.75760. [49] OTA Y, TAKAHASHI K, OTAKE S, et al. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition[J]. Oncotarget, 2018, 9( 23): 16400- 16417. DOI: 10.18632/oncotarget.24711. -



 PDF下载 ( 1357 KB)
PDF下载 ( 1357 KB)

 下载:
下载: