| [1] |
LI T, YAN Y, WANG B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis[J]. Stem Cells Dev, 2013, 22( 6): 845- 854. DOI: 10.1089/scd.2012.0395. |
| [2] |
Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2019)[J]. J Clin Hepatol, 2019, 35( 12): 2648- 2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007. |
| [3] |
LIU J, LIANG W, JING W, et al. Countdown to 2030: eliminating hepatitis B disease, China[J]. Bull World Health Organ, 2019, 97( 3): 230- 238. DOI: 10.2471/BLT.18.219469. |
| [4] |
LIU JW, XIE J, LI TS. Specific T-lymphocyte response and clinical outcome of patients with hepatitis B virus infection[J]. Med J Peking Union Med Coll Hosp, 2012, 3( 3): 350- 353. DOI: 10.3969/j.issn.1674-9081.2012.03.021. |
| [5] |
MA S, CHEN X, TAN Q, et al. An engineered novel lentivector specifically transducing dendritic cells and eliciting robust HBV-specific CTL response by upregulating autophagy in T cells[J]. Cell Cycle, 2018, 17( 10): 1220- 1234. DOI: 10.1080/15384101.2018.1471312. |
| [6] |
CHENG ST, REN JH, CAI XF, et al. HBx-elevated SIRT2 promotes HBV replication and hepatocarcinogenesis[J]. Biochem Biophys Res Commun, 2018, 496( 3): 904- 910. DOI: 10.1016/j.bbrc.2018.01.127. |
| [7] |
CHEN HY, CHEN ZX, HUANG RF, et al. Hepatitis B virus X protein activates human hepatic stellate cells through upregulating TGFβ1[J]. Genet Mol Res, 2014, 13( 4): 8645- 8656. DOI: 10.4238/2014.October.27.4. |
| [8] |
QIU H, LIN DY, LI JY. Screening and identification of dominant monoclonal HepG2 cell strain with 1.3-fold HBV genome[J]. World Chin J Dig, 2021, 29( 16): 934- 944. DOI: 10.11569/wcjd.v29.i16.934. |
| [9] |
National Health Commission. China health year book 2019[M]. Beijing: Peking Union Medical College Press, 2019.
国家卫生健康委员会. 2019 中国卫生健康统计年鉴[M]. 北京: 中国协和医科大学出版社, 2019.
|
| [10] |
ROCKEY DC. Liver fibrosis reversion after suppression of hepatitis B virus[J]. Clin Liver Dis, 2016, 20( 4): 667- 679. DOI: 10.1016/j.cld.2016.06.003. |
| [11] |
CHANG TT, LIAW YF, WU SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B[J]. Hepatology, 2010, 52( 3): 886- 893. DOI: 10.1002/hep.23785. |
| [12] |
TZIOMALOS K. Effect of antiviral treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis B[J]. World J Hepatol, 2010, 2( 3): 91- 93. DOI: 10.4254/wjh.v2.i3.91. |
| [13] |
CHEN BW, ZHU XJ, ZHANG X, et al. Influence of virologic response on disease progression in patients with compensated hepatitis B cirrhosis[J]. J Clin Hepatol, 2021, 37( 8): 1811- 1816. DOI: 10.3969/j.issn.1001-5256.2021.08.014. |
| [14] |
GIERSCH K, ALLWEISS L, VOLZ T, et al. Serum HBV pgRNA as a clinical marker for cccDNA activity[J]. J Hepatol, 2017, 66( 2): 460- 462. DOI: 10.1016/j.jhep.2016.09.028. |
| [15] |
MOHAMMADI A, TAJIK N, SHAH-HOSSEINI A, et al. FAS and FAS-ligand promoter polymorphisms in hepatitis B virus infection[J]. Hepat Mon, 2015, 15( 10): e26490. DOI: 10.5812/hepatmon.26490. |
| [16] |
KONDO Y, KOBAYASHI K, ASABE S, et al. Vigorous response of cytotoxic T lymphocytes associated with systemic activation of CD8 T lymphocytes in fulminant hepatitis B[J]. Liver Int, 2004, 24( 6): 561- 567. DOI: 10.1111/j.1478-3231.2004.0982.x. |
| [17] |
HAQUE M, LEI F, XIONG X, et al. Stem cell-derived viral antigen-specific T cells suppress HBV replication through production of IFN-γ and TNF-α[J]. iScience, 2020, 23( 7): 101333. DOI: 10.1016/j.isci.2020.101333. |
| [18] |
MOON B, CHANG S. Exosome as a delivery vehicle for cancer therapy[J]. Cells, 2022, 11( 3): 316. DOI: 10.3390/cells11030316. |
| [19] |
COCUCCI E, MELDOLESI J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles[J]. Trends Cell Biol, 2015, 25( 6): 364- 372. DOI: 10.1016/j.tcb.2015.01.004. |
| [20] |
JIA Y, LI Y, WANG YJ. Research progress on exosome targeted delivery of nucleic acid molecules[J]. China Med Herald, 2023, 20( 3): 33- 36, 49. DOI: 10.20047/j.issn1673-7210.2023.03.07. |
| [21] |
SUN JS, LU MY. Research progress on the formation of exosomes and their rolesin immune regulation[J]. J Tianjin Normal University(Natural Science Edition), 2020, 40( 1): 9- 15. DOI: 10.19638/j.issn1671-1114.20200102. |
| [22] |
|
| [23] |
ABDULKARIM AS, CAO H, HUANG B, et al. The large GTPase dynamin is required for hepatitis B virus protein secretion from hepatocytes[J]. J Hepatol, 2003, 38( 1): 76- 83. DOI: 10.1016/s0168-8278(02)00326-4. |



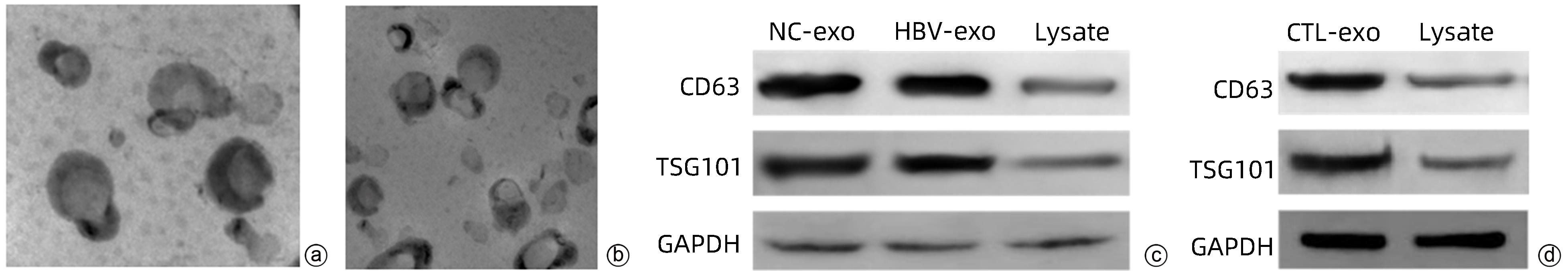




 DownLoad:
DownLoad: