| [1] |
Chinese Society of Hepatology, Chinese Medical Association. Experts opinon on expanding anti-HBV treatment for chronic hepatitis B[J]. Chin J Hepatol, 2022, 30( 2): 131- 136. DOI: 10.3760/cma.j.cn501113-20220209-00060. |
| [2] |
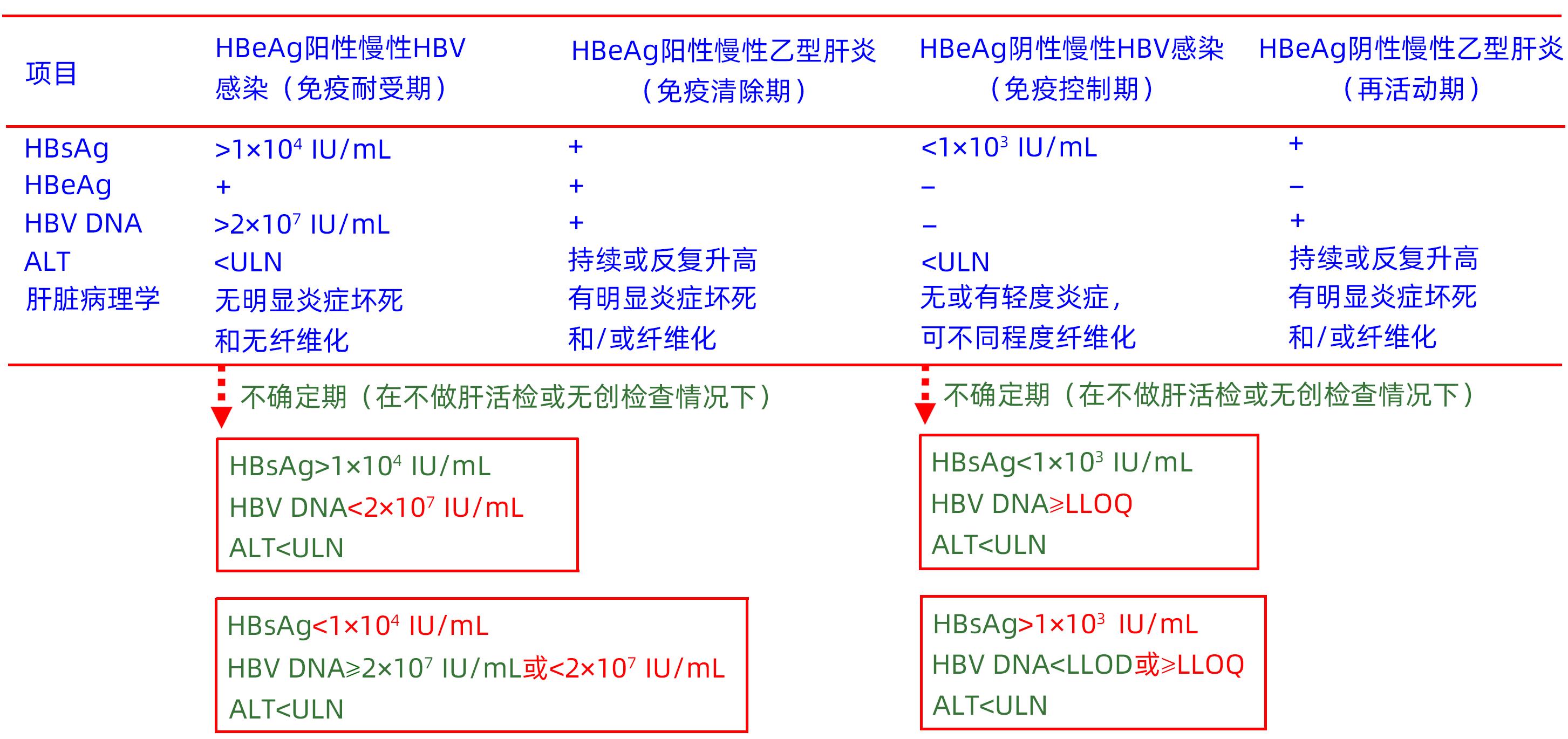
Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B(version 2022)[J]. Chin J Hepatol, 2022, 30( 12): 1309- 1331. DOI: 10.3760/cma.j.cn501113-20221204-00607. |
| [3] |
KUMAR M, SARIN SK, HISSAR S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT[J]. Gastroenterology, 2008, 134( 5): 1376- 1384. DOI: 10.1053/j.gastro.2008.02.075. |
| [4] |
HSU YN, PAN CQ, ABBASI A, et al. Clinical presentation and disease phases of chronic hepatitis B using conventional versus modified ALT criteria in Asian Americans[J]. Dig Dis Sci, 2014, 59( 4): 865- 871. DOI: 10.1007/s10620-014-3054-1. |
| [5] |
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection[J]. J Hepatol, 2017, 67( 2): 370- 398. DOI: 10.1016/j.jhep.2017.03.021. |
| [6] |
MARTIN P, NGUYEN MH, DIETERICH DT, et al. Treatment algorithm for managing chronic hepatitis B virus infection in the United States: 2021 update[J]. Clin Gastroenterol Hepatol, 2022, 20( 8): 1766- 1775. DOI: 10.1016/j.cgh.2021.07.036. |
| [7] |
CHOI HSJ, TONTHAT A, JANSSEN HLA, et al. Aiming for functional cure with established and novel therapies for chronic hepatitis B[J]. Hepatol Commun, 2022, 6( 5): 935- 949. DOI: 10.1002/hep4.1875. |
| [8] |
DUSHEIKO G, AGARWAL K, MAINI MK. New approaches to chronic hepatitis B[J]. N Engl J Med, 2023, 388( 1): 55- 69. DOI: 10.1056/NEJMra2211764. |
| [9] |
YAO KF, LIU JC, WANG J, et al. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone[J]. J Viral Hepat, 2021, 28( 7): 1025- 1033. DOI: 10.1111/jvh.13511. |
| [10] |
DUAN MH, CHI XL, XIAO HM, et al. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B[J]. Hepatol Int, 2021, 15( 2): 318- 327. DOI: 10.1007/s12072-021-10153-2. |
| [11] |
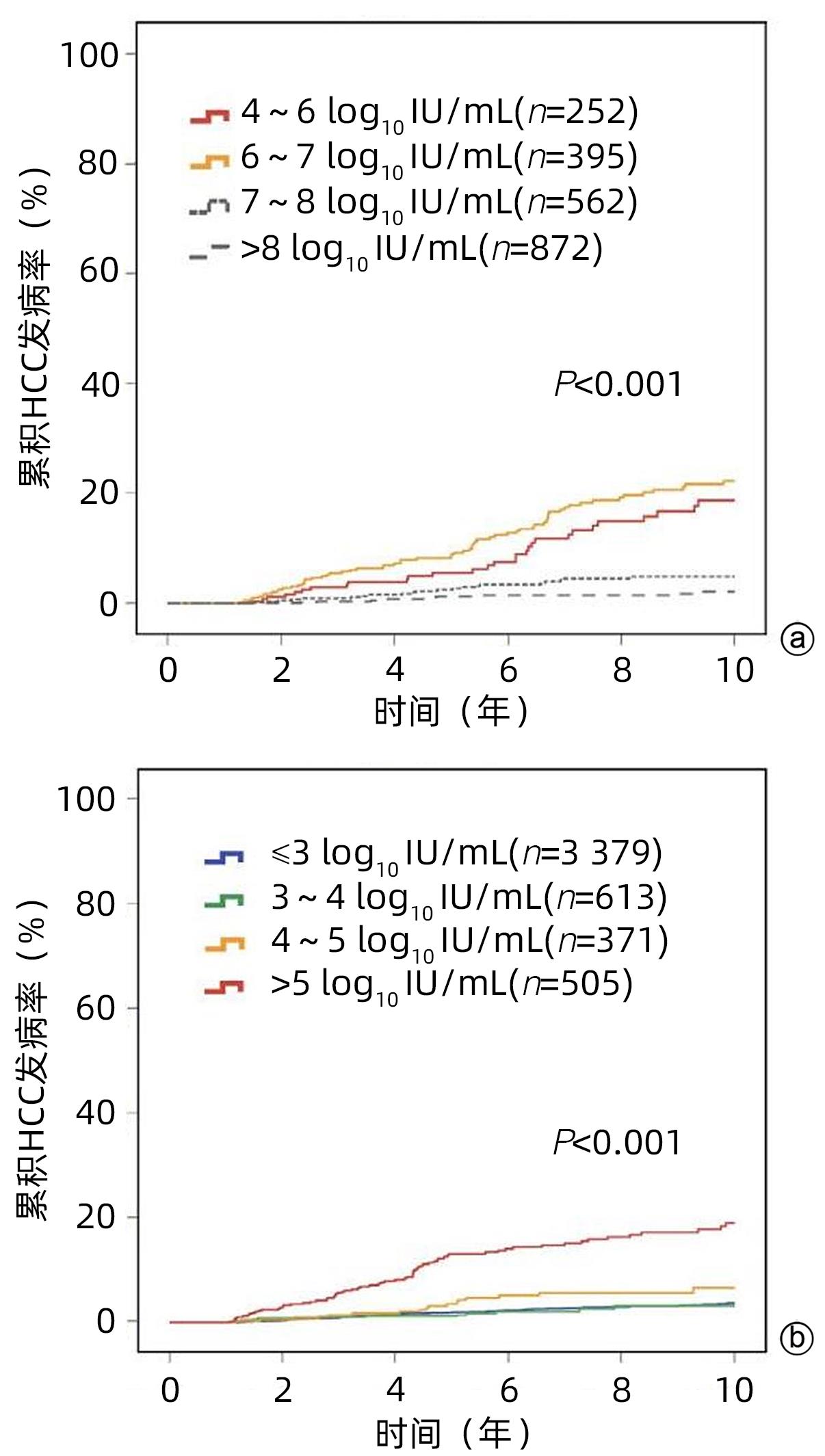
HUANG DQ, LI XH, LE MH, et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase[J]. Clin Gastroenterol Hepatol, 2022, 20( 8): 1803- 1812.e 5. DOI: 10.1016/j.cgh.2021.01.019. |
| [12] |
JIANG SW, LIAN X, HU AR, et al. Liver histopathological lesions is severe in patients with normal alanine transaminase and low to moderate hepatitis B virus DNA replication[J]. World J Gastroenterol, 2023, 29( 16): 2479- 2494. DOI: 10.3748/wjg.v29.i16.2479. |
| [13] |
WANG J, YAN XM, ZHU L, et al. Significant histological disease of patients with chronic hepatitis B virus infection in the grey zone[J]. Aliment Pharmacol Ther, 2023, 57( 5): 464- 474. DOI: 10.1111/apt.17272. |
| [14] |
XU XQ, WANG H, SHAN S, et al. The impact of the definitions of clinical phases on the profiles of grey-zone patients with chronic hepatitis B virus infection[J]. Viruses, 2023, 15( 5): 1212. DOI: 10.3390/v15051212. |
| [15] |
JU YH, HAN GR, ZHANG P, et al. Staging and clinical characteristics of pregnant women with chronic hepatitis B virus infection: A retrospective cohort study from Nanjing, China[J]. J Obstet Gynaecol Res, 2023, 49( 10): 2427- 2435. DOI: 10.1111/jog.15753. |
| [16] |
LIU JC, WANG J, YAN XM, et al. Presence of liver inflammation in Asian patients with chronic hepatitis B with normal ALT and detectable HBV DNA in absence of liver fibrosis[J]. Hepatol Commun, 2022, 6( 4): 855- 866. DOI: 10.1002/hep4.1859. |
| [17] |
CHOI GH, KIM GA, CHOI J, et al. High risk of clinical events in untreated HBeAg-negative chronic hepatitis B patients with high viral load and no significant ALT elevation[J]. Aliment Pharmacol Ther, 2019, 50( 2): 215- 226. DOI: 10.1111/apt.15311. |
| [18] |
KIM GA, HAN S, CHOI GH, et al. Moderate levels of serum hepatitis B virus DNA are associated with the highest risk of hepatocellular carcinoma in chronic hepatitis B patients[J]. Aliment Pharmacol Ther, 2020, 51( 11): 1169- 1179. DOI: 10.1111/apt.15725. |
| [19] |
TENG W, CHANG TT, YANG HI, et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines[J]. Hepatol Int, 2021, 15( 6): 1421- 1430. DOI: 10.1007/s12072-021-10263-x. |
| [20] |
SARIN SK, KUMAR M, LAU GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update[J]. Hepatol Int, 2016, 10( 1): 1- 98. DOI: 10.1007/s12072-015-9675-4. |
| [21] |
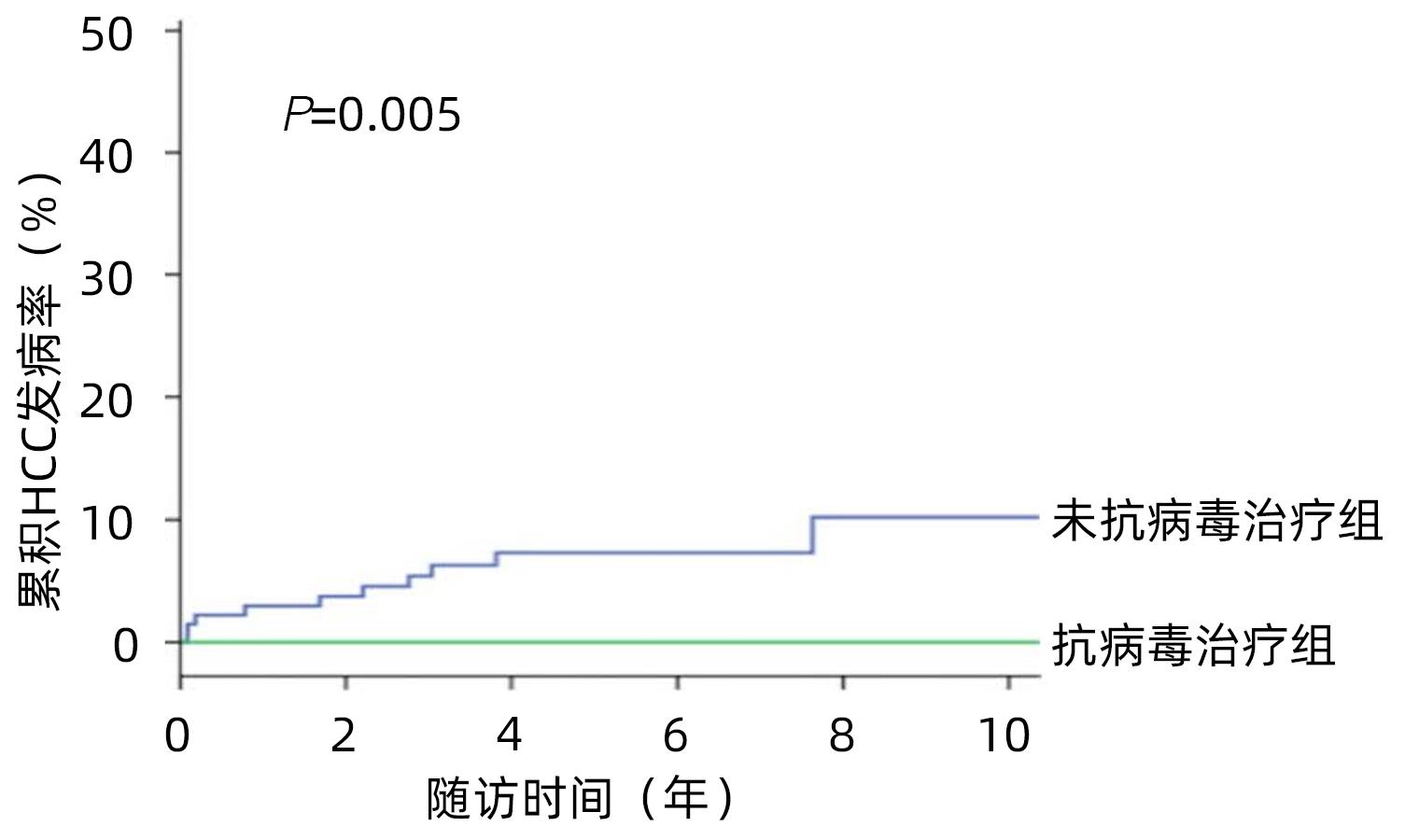
HUANG DQ, TRAN A, YEH ML, et al. Antiviral therapy substantially reduces HCC risk in patients with chronic hepatitis B infection in the indeterminate phase[J]. Hepatology, 2023, 78( 5): 1558- 1568. DOI: 10.1097/HEP.0000000000000459. |







 DownLoad:
DownLoad: