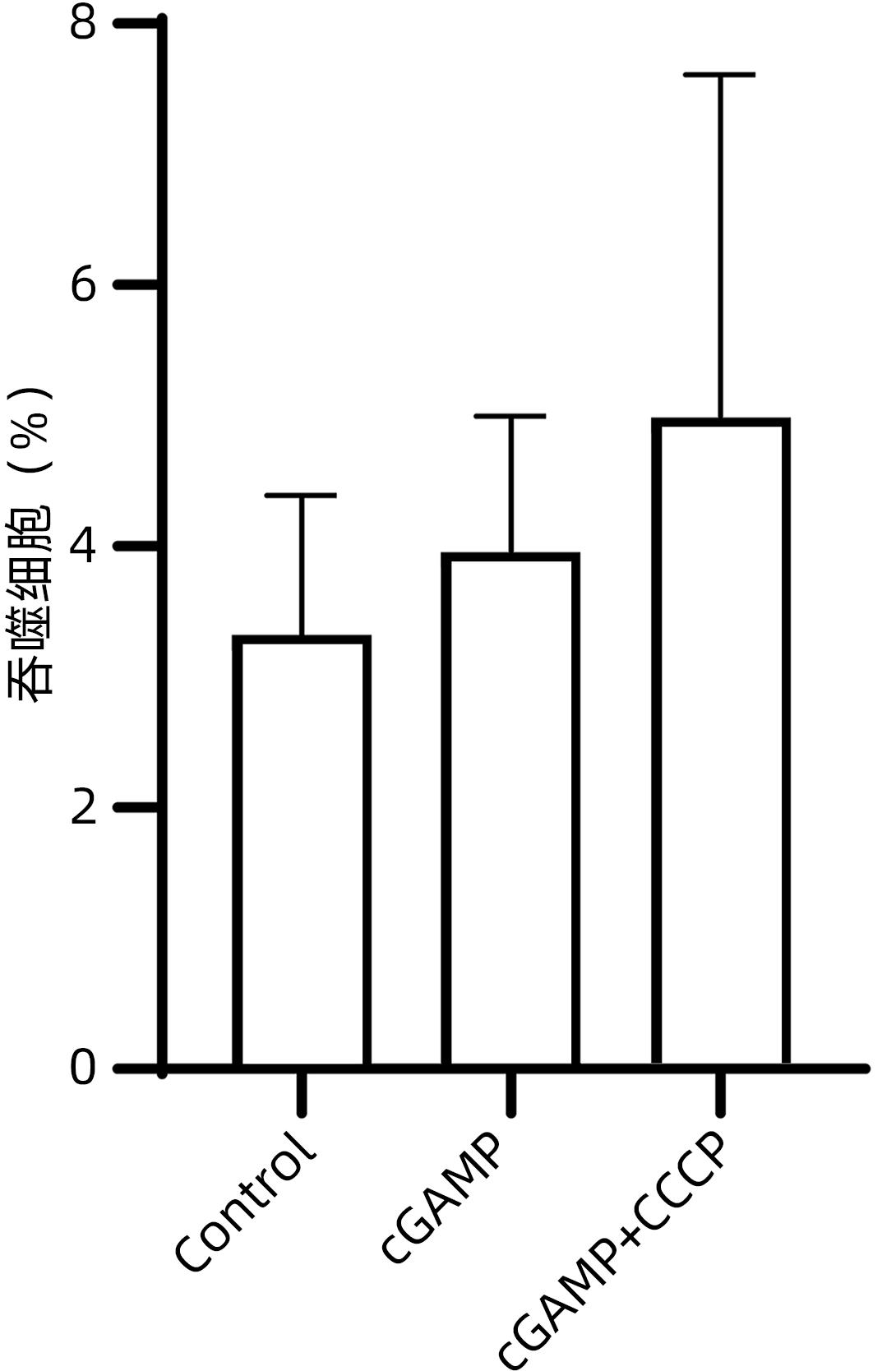
| [1] |
ALBILLOS A, LARIO M, ÁLVAREZ-MON M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance[J]. J Hepatol, 2014, 61( 6): 1385- 1396. DOI: 10.1016/j.jhep.2014.08.010. |
| [2] |
RACANELLI V, REHERMANN B. The liver as an immunological organ[J]. Hepatology, 2006, 43(2 Suppl 1): S54-S62. DOI: 10.1002/hep.21060. |
| [3] |
TILG H, VOGEL W, WIEDERMANN CJ, et al. Circulating interleukin-1 and tumor necrosis factor antagonists in liver disease[J]. Hepatology, 1993, 18( 5): 1132- 1138.
|
| [4] |
ERIKSSON AS, GRETZER C, WALLERSTEDT S. Elevation of cytokines in peritoneal fluid and blood in patients with liver cirrhosis[J]. Hepatogastroenterology, 2004, 51( 56): 505- 509.
|
| [5] |
SIEGAL FP, KADOWAKI N, SHODELL M, et al. The nature of the principal type 1 interferon-producing cells in human blood[J]. Science, 1999, 284( 5421): 1835- 1837. DOI: 10.1126/science.284.5421.1835. |
| [6] |
PAIJO J, DÖRING M, SPANIER J, et al. cGAS senses human cytomegalovirus and induces type I interferon responses in human monocyte-derived cells[J]. PLoS Pathog, 2016, 12( 4): e1005546. DOI: 10.1371/journal.ppat.1005546. |
| [7] |
ZHANG C, SHANG G, GUI X, et al. Structural basis of STING binding with and phosphorylation by TBK1[J]. Nature, 2019, 567( 7748): 394- 398. DOI: 10.1038/s41586-019-1000-2. |
| [8] |
ISHIKAWA H, MA Z, BARBER GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity[J]. Nature, 2009, 461( 7265): 788- 792. DOI: 10.1038/nature08476. |
| [9] |
ABE T, BARBER GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1[J]. J Virol, 2014, 88( 10): 5328- 5341. DOI: 10.1128/JVI.00037-14. |
| [10] |
FITZGERALD KA, MCWHIRTER SM, FAIA KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway[J]. Nat Immunol, 2003, 4( 5): 491- 496. DOI: 10.1038/ni921. |
| [11] |
SMEDSRØD B, PERTOFT H, GUSTAFSON S, et al. Scavenger functions of the liver endothelial cell[J]. Biochem J, 1990, 266( 2): 313- 327. DOI: 10.1042/bj2660313. |
| [12] |
WILLEKENS FL, WERRE JM, KRUIJT JK, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors[J]. Blood, 2005, 105( 5): 2141- 2145. DOI: 10.1182/blood-2004-04-1578. |
| [13] |
GREGORY SH, SAGNIMENI AJ, WING EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils[J]. J Immunol, 1996, 157( 6): 2514- 2520.
|
| [14] |
MERRIMAN RB, TRAN TT. AASLD practice guidelines: The past, the present, and the future[J]. Hepatology, 2016, 63( 1): 31- 34. DOI: 10.1002/hep.28345. |
| [15] |
CHRYSAVGIS L, PAPATHEODORIDI A, CHOLONGITAS E, et al. Significance of circulating cell-free DNA species in non-alcoholic fatty liver disease[J]. Int J Mol Sci, 2021, 22( 16): 8849. DOI: 10.3390/ijms22168849. |
| [16] |
BLASI A, PATEL VC, ADELMEIJER J, et al. Plasma levels of circulating DNA are associated with outcome, but not with activation of coagulation in decompensated cirrhosis and ACLF[J]. JHEP Rep, 2019, 1( 3): 179- 187. DOI: 10.1016/j.jhepr.2019.06.002. |
| [17] |
KHANAM A, CHUA JV, KOTTILIL S. Immunopathology of chronic hepatitis B infection: Role of innate and adaptive immune response in disease progression[J]. Int J Mol Sci, 2021, 22( 11): 5497. DOI: 10.3390/ijms22115497. |
| [18] |
RAZAVI H. Global epidemiology of viral hepatitis[J]. Gastroenterol Clin North Am, 2020, 49( 2): 179- 189. DOI: 10.1016/j.gtc.2020.01.001. |
| [19] |
TSENG TC, HUANG LR. Immunopathogenesis of hepatitis B virus[J]. J Infect Dis, 2017, 216( suppl_8): S765-S770. DOI: 10.1093/infdis/jix356. |
| [20] |
ASHARE A, STANFORD C, HANCOCK P, et al. Chronic liver disease impairs bacterial clearance in a human model of induced bacteremia[J]. Clin Transl Sci, 2009, 2( 3): 199- 205. DOI: 10.1111/j.1752-8062.2009.00122.x. |
| [21] |
BERNSMEIER C, TRIANTAFYLLOU E, BRENIG R, et al. CD14 + CD15 - HLA-DR - myeloid-derived suppressor cells impair antimicrobial responses in patients with acute-on-chronic liver failure[J]. Gut, 2018, 67( 6): 1155- 1167. DOI: 10.1136/gutjnl-2017-314184. |



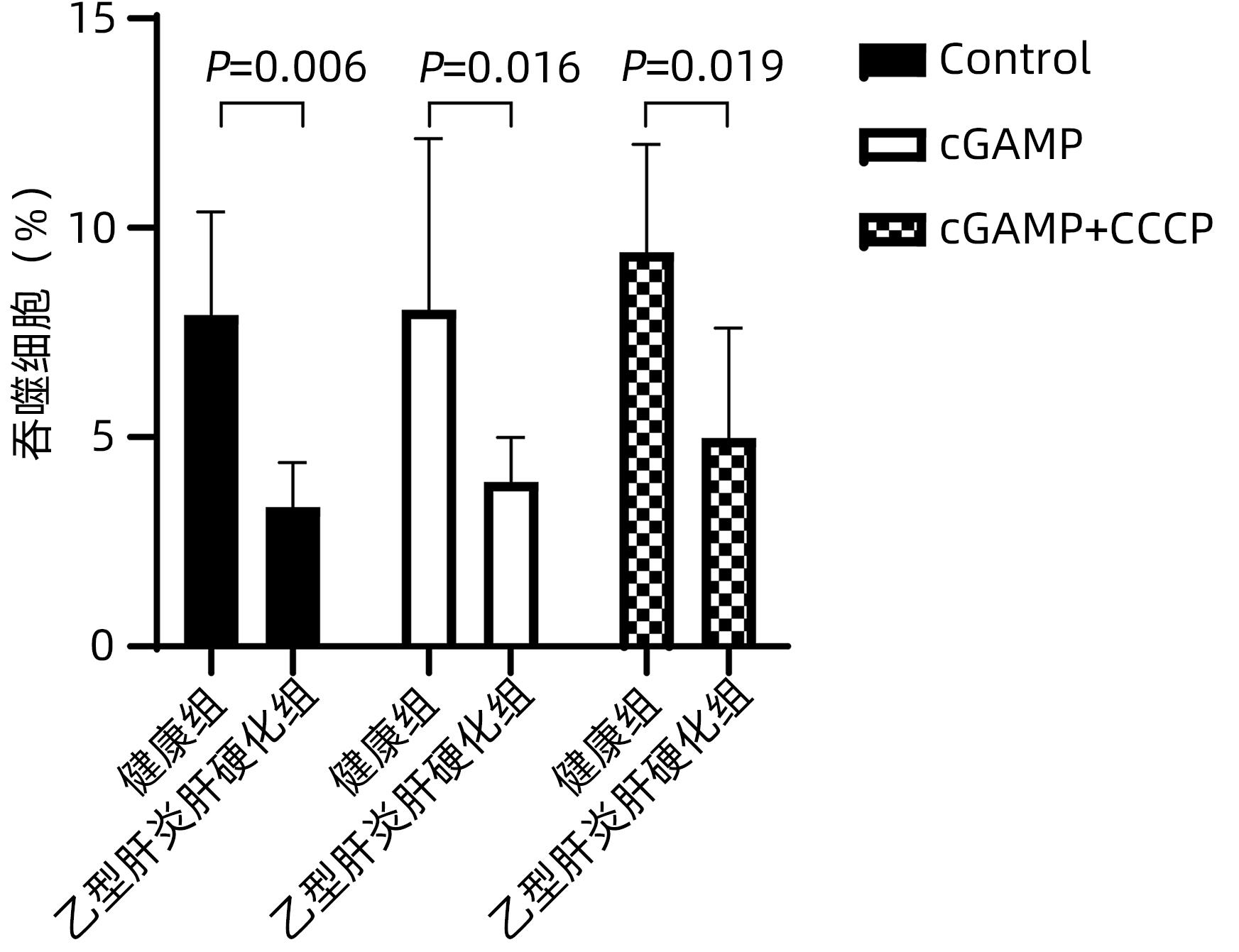




 DownLoad:
DownLoad: