| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [2] |
VILLANUEVA A. Hepatocellular carcinoma[J]. N Engl J Med, 2019, 380(15): 1450-1462. DOI: 10.1056/NEJMra1713263. |
| [3] |
VESSELLE G, QUIRIER-LELEU C, VELASCO S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma[J]. Eur Radiol, 2016, 26(6): 1640-1648. DOI: 10.1007/s00330-015-3982-y. |
| [4] |
SONG MJ, CHUN HJ, SONG DS, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma[J]. J Hepatol, 2012, 57(6): 1244-1250. DOI: 10.1016/j.jhep.2012.07.017. |
| [5] |
CAO B, ZHANG GW. Meta analysis of efficacy and safety of drug-eluting beads transarterial chemoembolization and conventional transarterial chemoembolization in the treatment of advanced hepatocellular carcinoma[J]. China Med Herald, 2021, 18(26): 95-99. https://www.cnki.com.cn/Article/CJFDTOTAL-YYCY202126023.htm |
| [6] |
SCARTOZZI M, BARONI GS, FALOPPI L, et al. Trans-arterial chemo-embolization (TACE), with either lipiodol (traditional TACE) or drug-eluting microspheres (precision TACE, pTACE) in the treatment of hepatocellular carcinoma: efficacy and safety results from a large mono-institutional analysis[J]. J Exp Clin Cancer Res, 2010, 29(1): 164. DOI: 10.1186/1756-9966-29-164. |
| [7] |
ZHOU GH, HAN J, SUN JH, et al. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres Ⓡ beads in Chinese hepatocellular carcinoma patients[J]. BMC Cancer, 2018, 18(1): 644. DOI: 10.1186/s12885-018-4566-4. |
| [8] |
SUN J, ZHOU G, XIE X, et al. Efficacy and safety of drug-eluting beads transarterial chemoembolization by callispheres Ⓡ in 275 hepatocellular carcinoma patients: Results from the Chinese CalliSpheres Ⓡ Transarterial Chemoembolization in Liver Cancer (CTILC) Study[J]. Oncol Res, 2020, 28(1): 75-94. DOI: 10.3727/096504019X15662966719585. |
| [9] |
KONG J, KONG J, PAN B, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA[J]. PLoS One, 2012, 7(5): e37266. DOI: 10.1371/journal.pone.0037266. |
| [10] |
QIN S, LI Q, GU S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial[J]. Lancet Gastroenterol Hepatol, 2021, 6(7): 559-568. DOI: 10.1016/S2468-1253(21)00109-6. |
| [11] |
ZHAO S, ZHANG T, DOU W, et al. A comparison of transcatheter arterial chemoembolization used with and without apatinib for intermediate-to advanced-stage hepatocellular carcinoma: a systematic review and meta-analysis[J]. Ann Transl Med, 2020, 8(8): 542. DOI: 10.21037/atm.2020.02.125. |
| [12] |
RIMASSA L, PRESSIANI T, MERLE P. Systemic treatment options in hepatocellular carcinoma[J]. Liver Cancer, 2019, 8(6): 427-446. DOI: 10.1159/000499765. |
| [13] |
FINN RS, ZHU AX. Evolution of systemic therapy for hepatocellular carcinoma[J]. Hepatology, 2021, 73(Suppl 1): 150-157. DOI: 10.1002/hep.31306. |
| [14] |
|
| [15] |
XU J, SHEN J, GU S, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase Ⅱ trial[J]. Clin Cancer Res, 2021, 27(4): 1003-1011. DOI: 10.1158/1078-0432.CCR-20-2571. |
| [16] |
JU S, WANG W, CHEN P, et al. Drug-eluting bead transarterial chemoembolization followed by apatinib is effective and safe in treating hepatocellular carcinoma patients with BCLC stage C[J]. Clin Res Hepatol Gastroenterol, 2022, 46(3): 101859. DOI: 10.1016/j.clinre.2022.101859. |
| [17] |
GRETEN TF, MAUDA-HAVAKUK M, HEINRICH B, et al. Combined locoregional-immunotherapy for liver cancer[J]. J Hepatol, 2019, 70(5): 999-1007. DOI: 10.1016/j.jhep.2019.01.027. |
| [18] |
Bureau of Medical Administration, National Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. J Clin Hepatol, 2020, 36(2): 277-292. DOI: 10.3969/j.issn.1001-5256.2020.02.007. |
| [19] |
LLOVET JM, LENCIONI R. mRECIST for HCC: Performance and novel refinements[J]. J Hepatol, 2020, 72(2): 288-306. DOI: 10.1016/j.jhep.2019.09.026. |
| [20] |
KUDO M, UESHIMA K, IKEDA M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial[J]. Gut, 2020, 69(8): 1492-1501. DOI: 10.1136/gutjnl-2019-318934. |
| [21] |
QIU Z, SHEN L, JIANG Y, et al. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study[J]. Ann Transl Med, 2021, 9(4): 283. DOI: 10.21037/atm-20-5360. |
| [22] |
PENG S, ZHANG Y, PENG H, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by Apatinib[J]. Cancer Lett, 2016, 373(2): 193-202. DOI: 10.1016/j.canlet.2016.01.015. |
| [23] |
KAN X, LIANG B, ZHOU G, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: A propensity score matching analysis[J]. Front Oncol, 2020, 10: 970. DOI: 10.3389/fonc.2020.00970. |
| [24] |
POL J, VACCHELLI E, ARANDA F, et al. Trial watch: Immunogenic cell death inducers for anticancer chemotherapy[J]. Oncoimmunology, 2015, 4(4): e1008866. DOI: 10.1080/2162402X.2015.1008866. |
| [25] |
MOTZ GT, SANTORO SP, WANG LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors[J]. Nat Med, 2014, 20(6): 607-615. DOI: 10.1038/nm.3541. |
| [26] |
VORON T, COLUSSI O, MARCHETEAU E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8 + T cells in tumors[J]. J Exp Med, 2015, 212(2): 139-148. DOI: 10.1084/jem.20140559. |
| [27] |
BRUIX J, QIN S, MERLE P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 389(10064): 56-66. DOI: 10.1016/S0140-6736(16)32453-9. |
| [28] |
JU S, ZHOU C, YANG C, et al. Apatinib plus camrelizumab with/without chemoembolization for hepatocellular carcinoma: A real-world experience of a single center[J]. Front Oncol, 2021, 11: 835889. DOI: 10.3389/fonc.2021.835889. |
| [29] |
LIU Y, CHEN X, GAO X, et al. Apatinib-induced hyperammonemic encephalopathy[J]. J Oncol Pharm Pract, 2020, 26(2): 465-470. DOI: 10.1177/1078155219846253. |
| [30] |
LARKIN J, HODI FS, WOLCHOK JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma[J]. N Engl J Med, 2015, 373(13): 1270-1271. DOI: 10.1056/NEJMc1509660. |
| [31] |
HODI FS, BALLINGER M, LYONS B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy[J]. J Clin Oncol, 2018, 36(9): 850-858. DOI: 10.1200/JCO.2017.75.1644. |



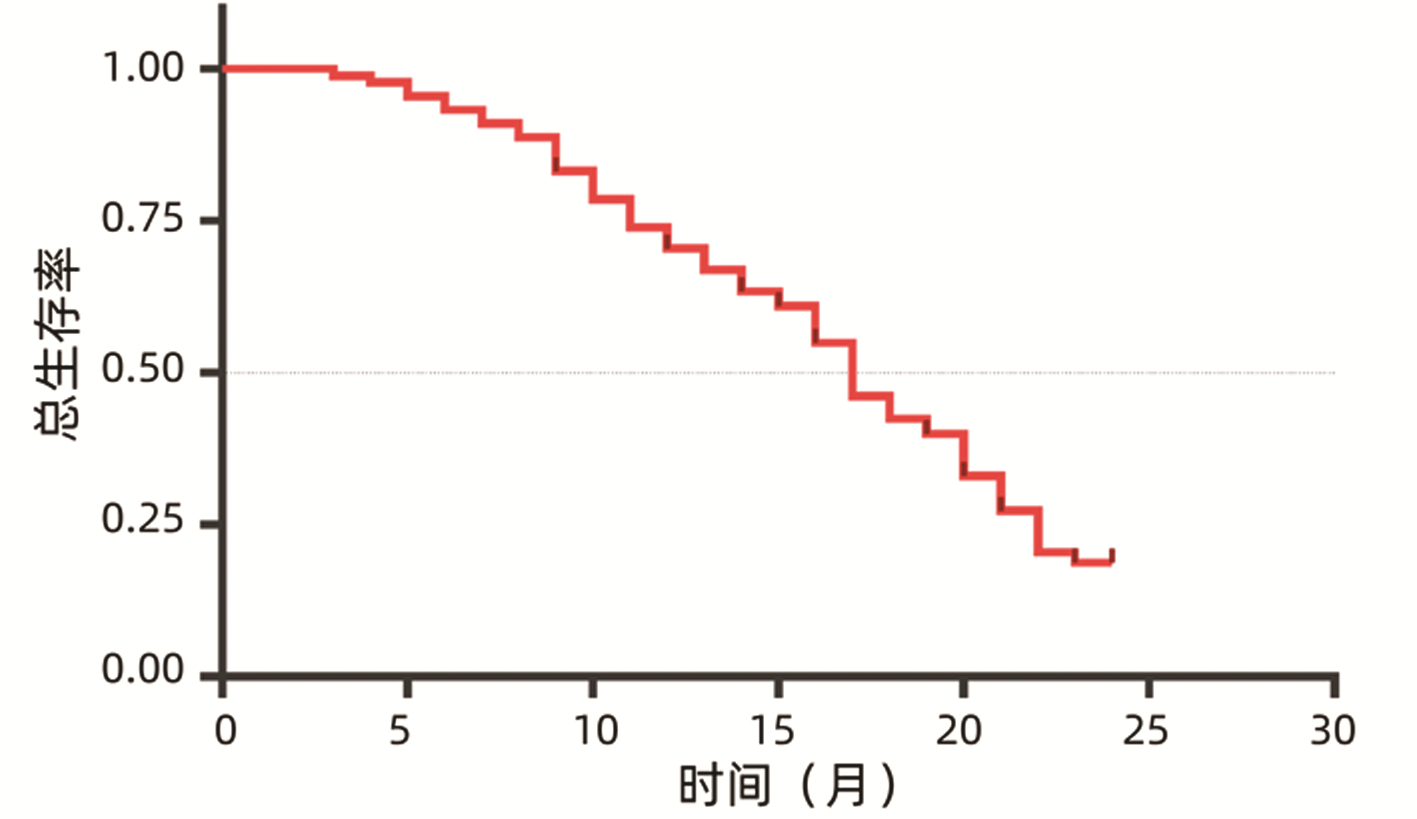




 DownLoad:
DownLoad:
