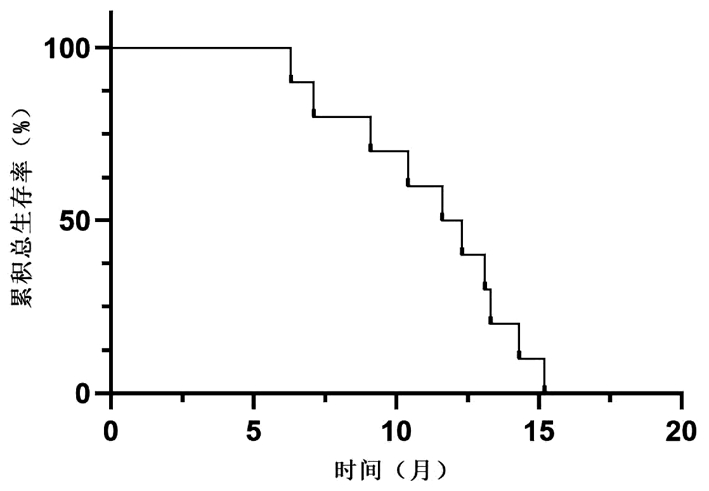
| [1] |
SUNG H, FERLAY J, SIEGEL RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. DOI: 10.3322/caac.21660. |
| [2] |
NENU I, BREABAN I, PASCALAU S, et al. The future is now: beyond first line systemic therapy in hepatocellular carcinoma[J]. Transl Cancer Res, 2019, 8(Suppl 3): S261-S274. DOI: 10.21037/tcr.2018.11.23. |
| [3] |
XUE X, LIAO W, XING Y. Comparison of clinical features and outcomes between HBV-related and non-B non-C hepatocellular carcinoma[J]. Infect Agent Cancer, 2020, 15: 11. DOI: 10.1186/s13027-020-0273-2. |
| [4] |
BSISU I, RMILAH AA. Global elimination of chronic hepatitis[J]. N Engl J Med, 2019, 381(6): 589. DOI: 10.1056/NEJMc1908197. |
| [5] |
SEKO Y, YAMAGUCHI K, ITOH Y. The genetic backgrounds in nonalcoholic fatty liver disease[J]. Clin J Gastroenterol, 2018, 11(2): 97-102. DOI: 10.1007/s12328-018-0841-9. |
| [6] |
MALUCCIO M, COVEY A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma[J]. CA Cancer J Clin, 2012, 62(6): 394-399. DOI: 10.3322/caac.21161. |
| [7] |
CHUNG SY, KIM KJ, SEONG J. Biomarkers for locally advanced hepatocellular carcinoma patients treated with liver-directed combined radiotherapy[J]. Liver Cancer, 2022, 11(3): 247-255. DOI: 10.1159/000522000. |
| [8] |
FINN RS, QIN S, IKEDA M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma[J]. N Engl J Med, 2020, 382(20): 1894-1905. DOI: 10.1056/NEJMoa1915745. |
| [9] |
EL-KHOUEIRY AB, SANGRO B, YAU T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial[J]. Lancet, 2017, 389(10088): 2492-2502. DOI: 10.1016/S0140-6736(17)31046-2. |
| [10] |
ZHU AX, FINN RS, EDELINE J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial[J]. Lancet Oncol, 2018, 19(7): 940-952. DOI: 10.1016/S1470-2045(18)30351-6. |
| [11] |
PFISTER D, NÚÑEZ NG, PINYOL R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC[J]. Nature, 2021, 592(7854): 450-456. DOI: 10.1038/s41586-021-03362-0. |
| [12] |
JI F, NGUYEN MH. Cabozantinib plus atezolizumab in advanced hepatocellular carcinoma and the role of adjuvant antiviral therapy[J]. Lancet Oncol, 2022, 23(8): 962-963. DOI: 10.1016/S1470-2045(22)00383-7. |
| [13] |
KELLEY RK, RIMASSA L, CHENG AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial[J]. Lancet Oncol, 2022, 23(8): 995-1008. DOI: 10.1016/S1470-2045(22)00326-6. |
| [14] |
LENCIONI R, LLOVET JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. DOI: 10.1055/s-0030-1247132. |
| [15] |
European Association for the Study of the Liver. Corrigendum to "EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma"[J Hepatol 69 (2018) 182-236][J]. J Hepatol, 2019, 70(4): 817. DOI: 10.1016/j.jhep.2019.01.020. |
| [16] |
KUDO M, MATILLA A, SANTORO A, et al. CheckMate 040 cohort 5: A phase Ⅰ/Ⅱ study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis[J]. J Hepatol, 2021, 75(3): 600-609. DOI: 10.1016/j.jhep.2021.04.047. |
| [17] |
WANG F, QIN S, SUN X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial[J]. J Hematol Oncol, 2020, 13(1): 47. DOI: 10.1186/s13045-020-00886-2. |
| [18] |
ONZI G, MORETTI F, BALBINOT S S, et al. Hepatocellular carcinoma in non-alcoholic fatty liver disease with and without cirrhosis[J]. Hepato Res, 2019, 5: 7. DOI: 10.20517/2394-5079.2018.114. |
| [19] |
DEGASPERI E, COLOMBO M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease[J]. Lancet Gastroenterol Hepatol, 2016, 1(2): 156-164. DOI: 10.1016/S2468-1253(16)30018-8. |
| [20] |
XU W, ZHANG X, WU JL, et al. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress[J]. J Hepatol, 2017, 67(2): 310-320. DOI: 10.1016/j.jhep.2017.03.017. |
| [21] |
SHEN ZQ, CHEN YF, CHEN JR, et al. CISD2 haploinsufficiency disrupts calcium homeostasis, causes nonalcoholic fatty liver disease, and promotes hepatocellular carcinoma[J]. Cell Rep, 2017, 21(8): 2198-2211. DOI: 10.1016/j.celrep.2017.10.099. |
| [22] |
SCHUSTER S, CABRERA D, ARRESE M, et al. Triggering and resolution of inflammation in NASH[J]. Nat Rev Gastroenterol Hepatol, 2018, 15(6): 349-364. DOI: 10.1038/s41575-018-0009-6. |
| [23] |
BOLAND P, WU J. Systemic therapy for hepatocellular carcinoma: beyond sorafenib[J]. Chin Clin Oncol, 2018, 7(5): 50. DOI: 10.21037/cco.2018.10.10. |
| [24] |
BENSON AB, D'ANGELICA MI, ABBOTT DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(5): 541-565. DOI: 10.6004/jnccn.2021.0022. |
| [25] |
General Office of National Health Commission. Standard for diagnosis and treatment of primary liver cancer (2022 edition)[J]. J Clin Hepatol, 2022, 38(2): 288-303. DOI: 10.3969/j.issn.1001-5256.2022.02.009. |
| [26] |
RODERBURG C, WREE A, DEMIR M, et al. The role of the innate immune system in the development and treatment of hepatocellular carcinoma[J]. Hepat Oncol, 2020, 7(1): HEP17. DOI: 10.2217/hep-2019-0007. |
| [27] |
QIN S, REN Z, MENG Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4): 571-580. DOI: 10.1016/S1470-2045(20)30011-5. |
| [28] |
CHEN J, HU X, LI Q, et al. Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients[J]. Ann Transl Med, 2020, 8(18): 1187. DOI: 10.21037/atm-20-6063. |



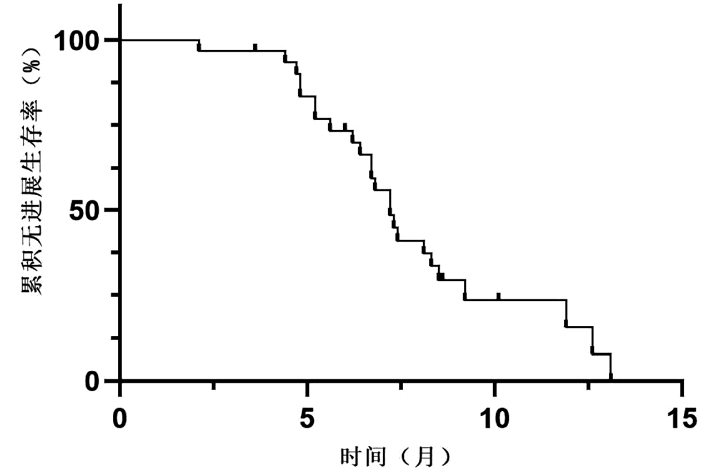




 DownLoad:
DownLoad: