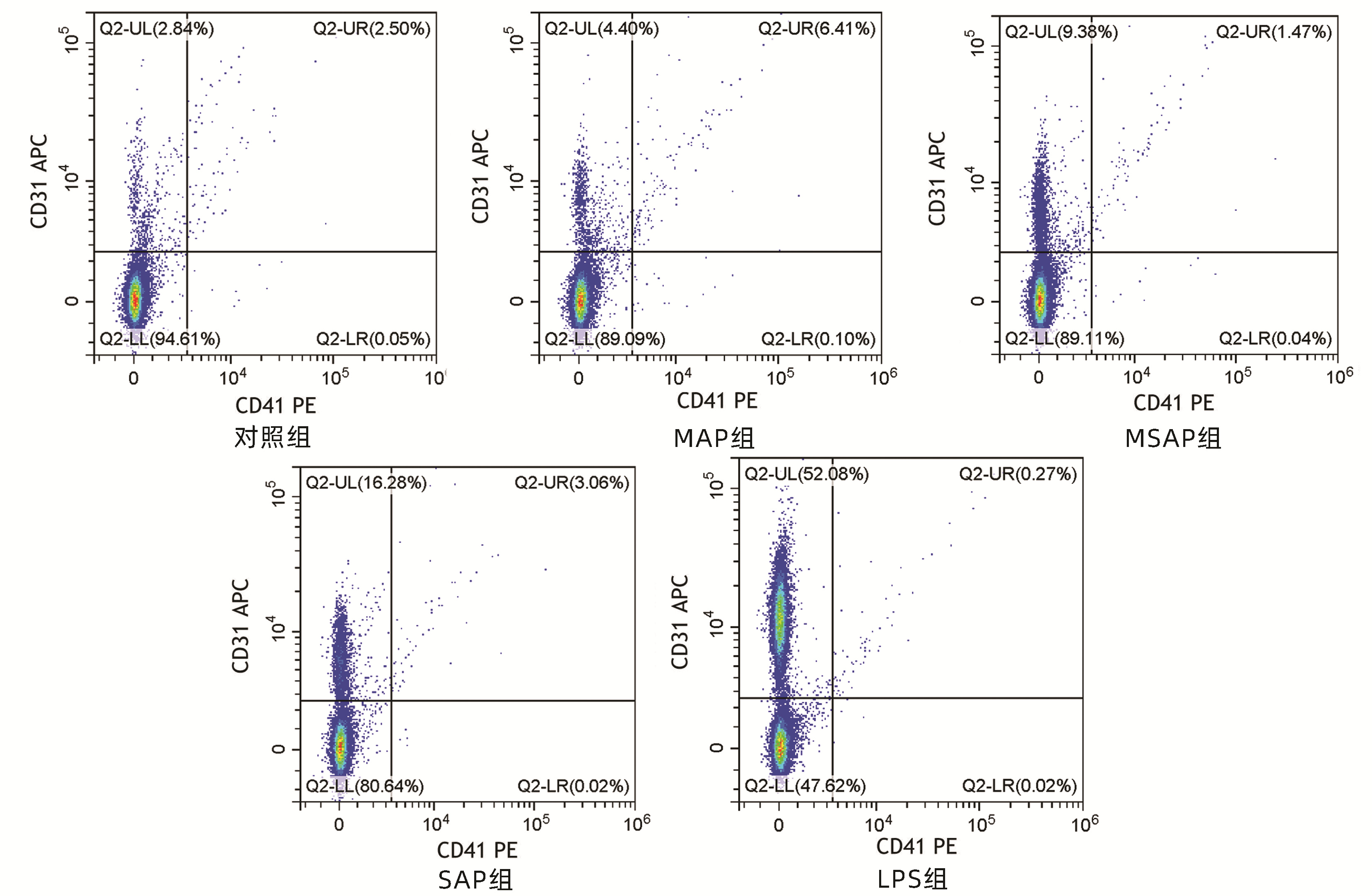
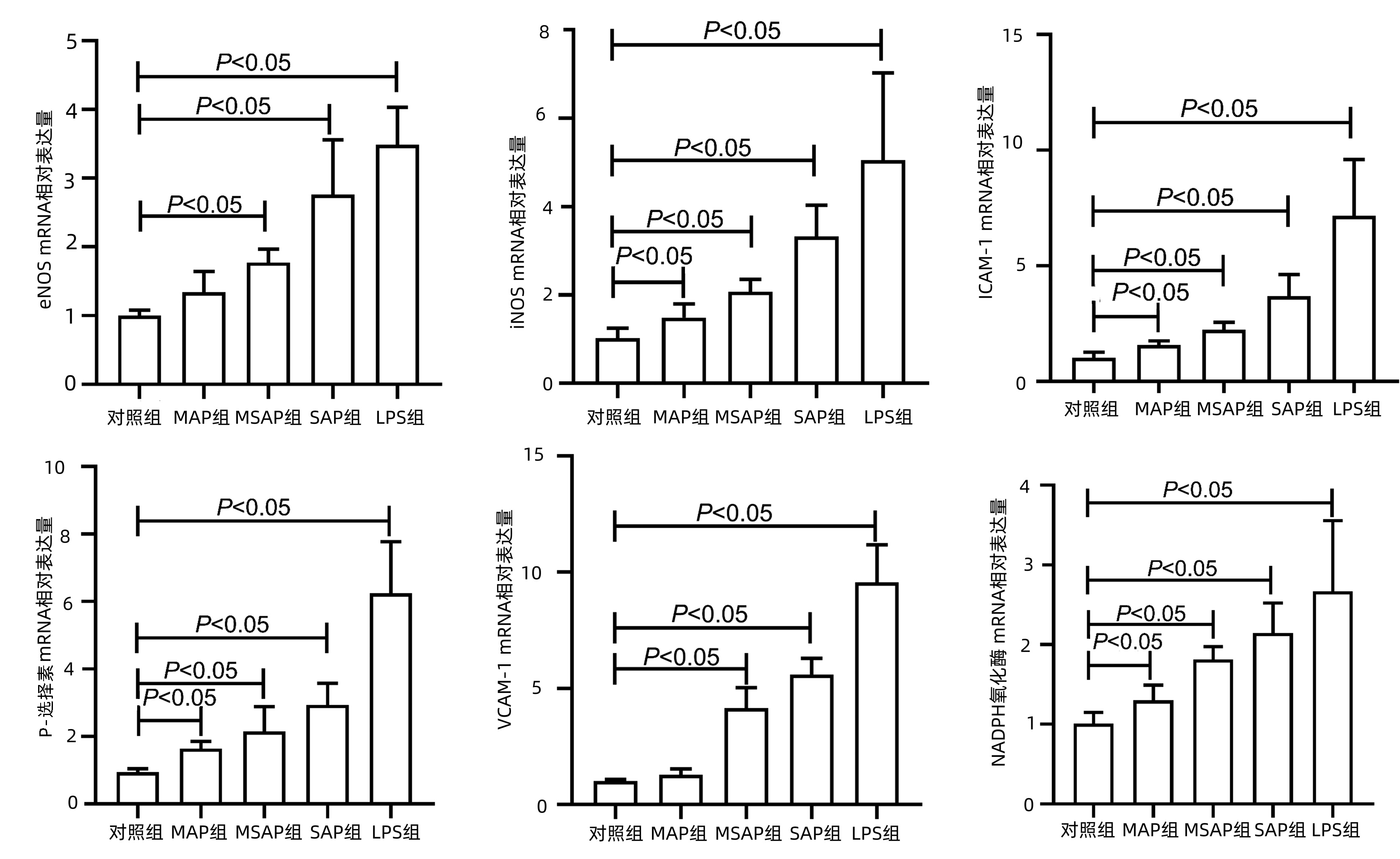
| [1] |
GARG PK, SINGH VP. Organ failure due to systemic injury in acute pancreatitis[J]. Gastroenterology, 2019, 156(7): 2008-2023. DOI: 10.1053/j.gastro.2018.12.041. |
| [2] |
SINGH P, GARG PK. Pathophysiological mechanisms in acute pancreatitis: Current understanding[J]. Indian J Gastroenterol, 2016, 35(3): 153-166. DOI: 10.1007/s12664-016-0647-y. |
| [3] |
GE N, XIA Q, YANG ZH, et al. Vascular endothelial injury and apoptosis in rats with severe acute pancreatitis[J]. Gastroenterol Res Pract, 2015, 2015: 235017. DOI: 10.1155/2015/235017. |
| [4] |
DUMNICKA P, MADUZIA D, CERANOWICZ P, et al. The interplay between inflammation, coagulation and endothelial injury in the early phase of acute pancreatitis: clinical implications[J]. Int J Mol Sci, 2017, 18(2): 354. DOI: 10.3390/ijms18020354. |
| [5] |
de OLIVEIRA C, KHATUA B, NOEL P, et al. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation[J]. J Clin Invest, 2020, 130(4): 1931-1947. DOI: 10.1172/JCI132767. |
| [6] |
MAHMOUD AM, WILKINSON FL, MCCARTHY EM, et al. Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress[J]. FASEB J, 2017, 31(10): 4636-4648. DOI: 10.1096/fj.201601244RR. |
| [7] |
PARKER B, Al-HUSAIN A, PEMBERTON P, et al. Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus[J]. Ann Rheum Dis, 2014, 73(6): 1144-1150. DOI: 10.1136/annrheumdis-2012-203028. |
| [8] |
JALAL D, RENNER B, LASKOWSKI J, et al. Endothelial microparticles and systemic complement activation in patients with chronic kidney disease[J]. J Am Heart Assoc, 2018, 7(14): e007818. DOI: 10.1161/JAHA.117.007818. |
| [9] |
ABBAS M, JESEL L, AUGER C, et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity: Role of the Ang Ⅱ/AT1 receptor/NADPH oxidase-mediated activation of MAPKs and PI3-kinase pathways[J]. Circulation, 2017, 135(3): 280-296. DOI: 10.1161/CIRCULATIONAHA.116.017513. |
| [10] |
Al-QAISSI A, PAPAGEORGIOU M, DESHMUKH H, et al. Effects of acute insulin-induced hypoglycaemia on endothelial microparticles in adults with and without type 2 diabetes[J]. Diabetes Obes Metab, 2019, 21(3): 533-540. DOI: 10.1111/dom.13548. |
| [11] |
BANKS PA, BOLLEN TL, DERVENIS C, et al. Classification of acute pancreatitis——2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-111. DOI: 10.1136/gutjnl-2012-302779. |
| [12] |
POHL PH, LOZITO TP, CUPERMAN T, et al. Catabolic effects of endothelial cell-derived microparticles on disc cells: Implications in intervertebral disc neovascularization and degeneration[J]. J Orthop Res, 2016, 34(8): 1466-1474. DOI: 10.1002/jor.23298. |
| [13] |
TOMKÖTTER L, ERBES J, TREPTE C, et al. The effects of pancreatic microcirculatory disturbances on histopathologic tissue damage and the outcome in severe acute pancreatitis[J]. Pancreas, 2016, 45(2): 248-253. DOI: 10.1097/MPA.0000000000000440. |
| [14] |
|
| [15] |
PERNOMIAN L, MOREIRA JD, GOMES MS. In the view of endothelial microparticles: Novel perspectives for diagnostic and pharmacological management of cardiovascular risk during diabetes distress[J]. J Diabetes Res, 2018, 2018: 9685205. DOI: 10.1155/2018/9685205. |
| [16] |
LI T, LUO N, DU L, et al. Tumor necrosis factor-α plays an initiating role in extracorporeal circulation-induced acute lung injury[J]. Lung, 2013, 191(2): 207-214. DOI: 10.1007/s00408-012-9449-x. |
| [17] |
CLEMMER JS, XIANG L, LU S, et al. Hyperglycemia-mediated oxidative stress increases pulmonary vascular permeability[J]. Microcirculation, 2016, 23(3): 221-229. DOI: 10.1111/micc.12267. |
| [18] |
BURGER D, TURNER M, XIAO F, et al. High glucose increases the formation and pro-oxidative activity of endothelial microparticles[J]. Diabetologia, 2017, 60(9): 1791-1800. DOI: 10.1007/s00125-017-4331-2. |
| [19] |
CAO WL, XIANG XH, CHEN K, et al. Potential role of NADPH oxidase in pathogenesis of pancreatitis[J]. World J Gastrointest Pathophysiol, 2014, 5(3): 169-177. DOI: 10.4291/wjgp.v5.i3.169. |
| [20] |
CHAN S, LIAN Q, CHEN MP, et al. Deferiprone inhibits iron overload-induced tissue factor bearing endothelial microparticle generation by inhibition oxidative stress induced mitochondrial injury, and apoptosis[J]. Toxicol Appl Pharmacol, 2018, 338: 148-158. DOI: 10.1016/j.taap.2017.11.005. |
| [21] |
CHEN YH, CHEN ZW, LI HM, et al. AGE/RAGE-induced EMP release via the NOX-derived ROS pathway[J]. J Diabetes Res, 2018, 2018: 6823058. DOI: 10.1155/2018/6823058. |



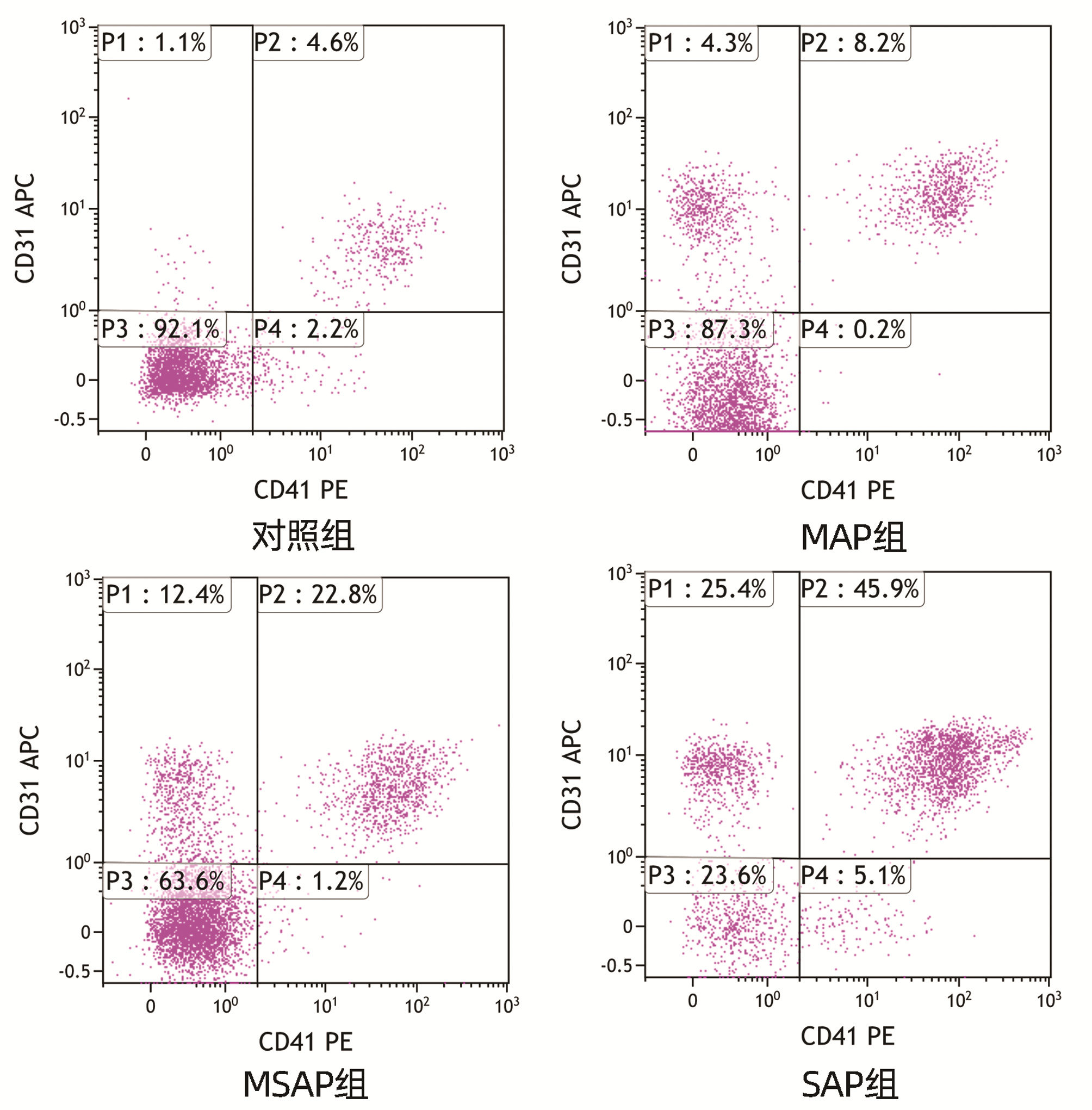




 DownLoad:
DownLoad: